Synthesis and Characterisation of Iron (II), Cobalt (II) and Nickel (II) Complexes Bearing a Novel Vanillin-Tryptophan Schiff Base as Prospective Antimicrobial Activities
Salisu Abubakar1*, Shallangwa GA2 and Ibrahim Abdulkadir2
1Department of Chemistry, Federal College of Education, Zaria, Nigeria
2Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
- *Corresponding Author:
- Salisu Abubakar
Department of Chemistry,
Federal College of Education,
Zaria,
Nigeria
Tel: 2 348030569234
E-mail: abubakarsalisu@abu.edu.ng
Received date: March 13, 2023, Manuscript No. IPJOIC-23-16099; Editor assigned date: March 15, 2023, PreQC No. IPJOIC-23-16099 (PQ); Reviewed date: March 29, 2023, QC No. IPJOIC-23-16099; Revised date: May 15, 2023, Manuscript No. IPJOIC-23-16099 (R); Published date: May 23, 2023, DOI: 10.36648/2472-1123.9.2.45
Citation: Abubakar S, Shallangwa GA, Abdulkadir I (2023) Synthesis and Characterisation of Iron (II), Cobalt (II) and Nickel (II) Complexes Bearing a Novel Vanillin-Tryptophan Schiff Base as Prospective Antimicrobial Activities. J Org Inorg Chem Vol:9 No:2.
Abstract
A novel Schiff base vanillin tryptophan ligand was synthesised and complexed with iron (II), cobalt (II) and nickel (II) ions in ethanol medium. They were characterised via elemental analysis, PXRD, FTIR, UV-visible spectroscopic, molar conductivity and magnetic susceptibility. The molar conductivity measurement suggested that the complexes are non-electrolytic in nature. The magnetic susceptibility and spectroscopic data suggested octahedral geometry for the coordinated compounds. Electronic absorption (λmas) for Fe2+ and Co2+ complexes are 480 nm and 520 nm respectively, while Ni2+ complex shows two bands at 480 and 570 nm, which signified the existence of n-* and -* transitions respectively. The PXRD technique shows that complexes are crystalline. Antimicrobial test results revealed that Co2+ and Fez2+ complexes have excellent activities against gram-negative bacteria (E. coli, F. shigella and S. typhi). They are all effective against gram-positive bacteria (MRSA, K. pneumniae and S. pneumonia) as well as fungi (A. niger and C. albicans).
Keywords
Schiff base; Complexes; Diffraction; Antimicrobial; Vanillin
Introduction
Schiff bases derived from amino acids and flavones like vanillin and quercetins, as well as their complexes, exhibit a wide range of biological activities [1]. The high affinity for the chelation of the Schiff bases towards the transition metals is utilised in preparing their solid complexes and due to its polyhydroxylated chemical structure, vanillin easily forms complexes with species that have free orbitals that may be occupied.
The potentials of vanillin Schiff base ligands in inorganic chemistry can be very well understood from the properties exhibited by their complexes, such as magnetics, luminescence, chirality, catalysis, cytotoxicity and ferro electricity [2]. Vanillin derivatives are found to be active against both gram positive and gram negative bacterial strains and have been shown to be effective against some fungal species.
L-Tryptophan (Trp) is an essential amino acid that is required for the biosynthesis of proteins in the body. It is very significant for nitrogen balance, maintenance of muscle mass and body weight in humans.
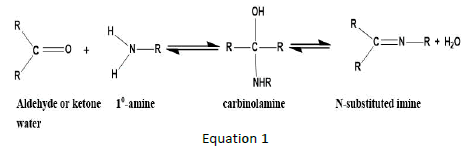
The electrophilic carbon atom in carbonyl compounds is nucleophilically attacked by amines in reactions that end in Schiff base ligands, creating a molecule with C=O substituted by C=N [3]. Depending on whether the carbonyl is an aldehyde or a ketone, the reaction is reversible.
Vanillin amine condensation products have biological activities as well as good complexation abilities with metal ions. This study focused on the synthesis, characterization, and testing for antimicrobial activities of a Schiff base ligand derived from the condensation of O-vanillin with L-tryptophan and complexes of their ligands with Fe2+, Co2+, and Ni2+ ions. Transition metal complexes with bidentate Schiff base ligands that contain nitrogen, oxygen or sulphur donor atoms contribute immensely to biological systems.
Numerous studies carried out on transition metal complexes derived from Schiff base ligands have pointed out their significance due to their properties and wider biological applications [4]. Kaczmarek MT, et al., Reported that Schiff reported that Schiff base complexes derived from 4-hydroxysalicylaldehyde and amines have strong anticancer activity. Schiff bases of ketones, their derivatives and their metal complexes are active anticancer and antioxidant agents. Transition metal ions are amongst the important species needed to keep human body healthy and active because several important biological functions depend upon their presence and their absence may lead to deficiency diseases. Opined that, most notable metals that exist in human body are in the form of ions such as: Fe2+, Co2+, Ni2+, Ca2+, Cu2+, Zn2+ and Cr2+. Transition metal complexes are observed to be generally more biologically active than the uncomplexed ligands, which are formed due to the presence of metal moieties [5].
Materials and Methods
All chemicals used for this research are of analytical grade (AR) and were obtained from sigma Aldrich and other reliable chemical vendors. The chemicals were used as purchased [6]. Mettler analytical weighing balance (MT 200B, 0.0001-200G), Jenway-7315 spectrophotometer, magnetic stirrer (Stuart), fume cupboard (Bio base), melting point machine (SMP 10 fascia, stuart bibby scientific), FTIR Spectrophotometer (nicolet iS5- thermo scientific), electrical conductivity meter (hanna instrument, HI 9835, woon socket R.I).
Synthesis of Schiff base ligand
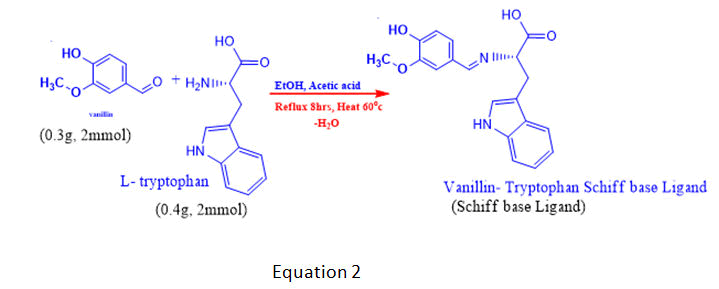
The method used to synthesise the ligand was adopted from that of Sani S, et al. with minor modifications. In 15 ml of ethanol, a solution of vanillin (0.30 g, 2 mmol) was mixed with a solution of l-tryptophan (0.4 g, 2 mmol) and 1 ml of dilute sodium hydroxide solution (0.056 g, 1 mmol). The reaction mixture was stirred for about 10 minutes and refluxed over a water bath for 5 hours at 60°C. The coloured precipitate that formed was filtered, washed with 50% cold ethanol and rinsed with diethyl ether [7]. It was allowed to dry in a vacuum desiccator to a constant weight, which gave a yield of 72.0% Schiff base Ligand (SL). The yellowish product was recrystallized in methanol for further purification.
Synthesis of the complexes
The complexes were prepared using standard methods of metal: Ligand ratio adopted from with some minor modifications. An ethanolic solution of Schiff base (20 mmol) was prepared and added drop wise to the solutions of metal (II) chloride (10.0 mmol), FeCl2, CoCl2 and NiCl2, which were stirred for about 20 minutes. The same concentrations were prepared and refluxed in a water bath at 60°C for 6 hours [8]. pH of the mixture was adjusted to 7.5 with a few drops of potassium hydroxide solution, and the coloured solids formed were filtered, washed with 50% cold ethanol, and finally dried in a vacuum desiccator to a constant weight. The synthetic routes are summarised in equations 2 and 3.

Physical measurements
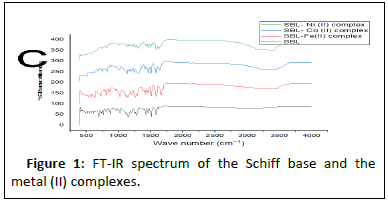
The solubilities of the Schiff base and metal complexes were checked in water, 50% ethanol and DMSO. The melting points of the complexes were also determined to establish their purity using an electrically heated melting point apparatus (SMP10, fascia, Stuart; bibby scientific) and molar conductivity was assessed to test the electrolytic nature of the complexes (with a hann scientific electrical conductivity meter) [9]. The wavelength of maximum absorption (max) of the Schiff base and the complexes was measured using an aqueous solution of the compounds in a UV/visible spectrophotometer (Jenway, 7315) in a 1 cm cuvette at 200 nm–780 nm in an FT-IR spectrometer (Nicolet iS5, thermo scientific). Elemental analysis was performed using the phase crystal analysis software and the report obtained from the PXRD data [10]. Magnetic susceptibility followed by an x-ray diffraction technique was used to determine the geometries, degree of crystalinity and morphological properties of the complexes (Figure 1).
Assessment of antimicrobial activities
Minimum Inhibitory Concentration (MIC): The MIC values were determined in accordance with method proposed by the clinical and laboratory standards institute using broth dilution techniques. McFarland's turbidity standard was developed and used in the study [11]. 1 ml of sterilised MH medium was added to each of the clean, dried and sterilised test tubes followed by 1 ml of 0.5 MCF bacterial suspension which contained about 1-2 108 CFU per mL of bacterial load. 1 mL of a 2000 g/mL solution of the test compounds was transferred into each test tube and serially diluted to 1000, 500, 250, 125, 62.5, 31.25 and 15.625 g/mL.
The solution was added to the mixture, followed by 0.1 mL of the bacterial suspension. The test tubes were incubated at 37°C for 24 hours to enhance bacterial growth. The tubes with the lowest and highest turbidity were carefully examined, compared with the standard, and the results were recorded.
Minimum Bactericidal Concentration (MBC): The MBC was assessed using the method developed by to ascertain whether the test pathogens were completely killed or just inhibited in their growth. Mueller Hinton agar was sterilised and poured into Petri dishes and the media was allowed to cool and solidify [12]. An aliquots samples that showed sensitivity to the MIC test were carefully selected and subjected to MBC evaluation. They were subculture and incubated at 28°C for 7–14 days in some cases. To determine CFU, the plates of the media were taken for microscopy and observed for bacterial growth.
Minimum Fungicidal Concentration (MFC): Khaidir HBSS, et al. method was adopted to carry out this test using micro broth dilution technique with little modi ications. Two fungal spp (Aspergillus Niger and Candida albicans) isolates were used, grown for 7 days at 28°C incubation period in potato dextrose agar [13]. The stock suspension was diluted with normal saline solution to corresponding concentration of 1 × 105 cfu/mL and viability of the fungi spp was con irmed using the sabouraud dextrose agar plates and counting the number of cfu/Ml.
Two fold serially diluted solution of the test compounds were prepared to obtained 200 μg/ml, 100 μg/ml, 50 μg/ml, 25 μg/ml and 12.5 μg/ml respectively. A micro pipette was used to transfer 0.1 ml of the test fungal strain solution into the test tubes containing the mixture with varied concentrations of the compounds. The mixture was incubated at 38°C for 72 hours a ter which the turbidity was observed and compared with standard for the growth of the pathogens [14]. The turbidity (growth) between the test tube which contained lowest and the highest concentration of the compound were examined and compared with standard. The results were carefully recorded in accordance with clinical and laboratory standard institute. Samples that showed sensitivity to the MIC test were carefully selected and subjected to MBC evaluation. They were sub cultured and incubated at 28°C for 7–14 days in some cases. To determine CFU, the plates of the media were taken for microscopy and observed for bacterial growth.
Results and Discussion
The results in Table 1 indicate the colour of the Schiff base and the metal complexes, percentage yields and solubilities in some solvents. It revealed that the Schiff base ligand is sparingly soluble in water but readily dissolved in DMSO and 50% ethanol solutions, while the complexes are soluble in water and DMSO, respectively [15]. It also indicated that the Schiff base has a lower melting point (84°C) than the complexes, but the Ni2+ complex has the highest melting temperature of >350°C, followed by the cobalt II complex (271°C) and the iron II complex (245°C). Molar conductivity measurement shows that both the ligand and the complexes were non electrolytic in nature, and magnetic susceptibility measurement obtained at room temperature revealed that the complexes are paramagnetic with observed magnetic moments as: Fe2+=5.73 B.M., Co2+=4.52 B.M and Ni2+=3.46 B.M., respectively, which are slightly higher than the spin only values due to unpaired electrons, suggesting an octahedral geometry for all the complexes. This is similar to the work of Urowska1 Z, et al. [16].
| Products | Colour | % Yield | Solubility | Mpt (°C) | Λm (Sm² mol−1) | µeff (B.M) |
|---|---|---|---|---|---|---|
| Vanillin-Tryptophan SBL | Golden yellow | 72 | SS (H2O) SL ( EtOH) SL DMSO) |
84 | 10300 | - |
| Vanillin-Tryptophan-Fe2+ | Greenish yellow | 85 | SL (H2O) SS (EtOH) SL (H2O) SS (EtOH) |
245 | 5000 | 5.73 |
| Vanillin-Tryptophan-Co2+ | Brownish yellow | 81 | SL (H2O) SL(EtOH) SL(DMSO) |
271 | 17300 | 4.52 |
| Vanillin-Tryptophan-Ni2+ | Yellowish-green | 59.4 | SS (H2O) SL (EtOH) SL(DMSO) |
>350 | 52900 | 3.46 |
Keys: SL: Soluble; SS: Sparingly Soluble |
||||||
Table 1: Shows some physical parameters test results for Schiff base and metal complexes.
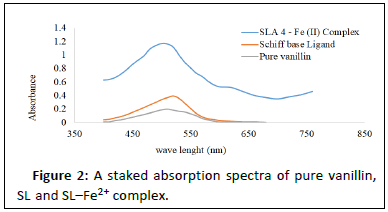
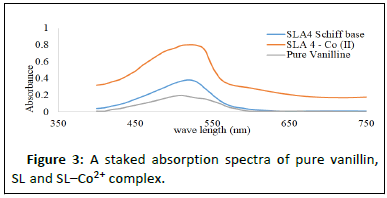
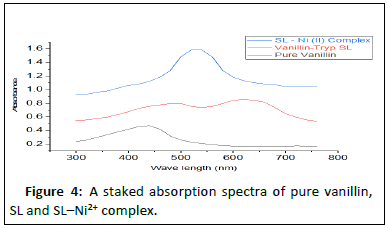
Results in Table 2 show electronic transitions within the Schiff base and the complexes scanned from 300 to 750 nm in ethanolic solutions, which indicate some bands as n- and -type transitions from nonbonding to anti bonding molecular orbitals and delocalization of pi-electrons within the benzene rings (Figures 2-4). The absorption bands assigned and the proposed geometries of the complexes are as follows: Fe2+ absorbs at 460-480 nm, Co2+ absorbs at 500-520 nm and Ni2+ absorbs at 369-480 and 550-570 nm, respectively. This result is in agreement with that of [17].
| S/No | Compounds | Wave length λmax (nm) | Transition assigned | Geometries |
|---|---|---|---|---|
1 |
Schiff base Ligand (SL) |
255–350 | π-π* (C=C in benzene) | ---- |
| 330–380 | n-π* (C=N in imine) | |||
| 2 | SL–Fe2+ complex | 460–480 | π-π* (C=C in benzene) | Octahedral |
| 3 | SL–Co2+ complex | 500–520 | n-π* (C=N in imine) | Octahedral |
4 |
SL–Ni2+ complex |
550–570 | π-π* (C=N in imine) | Octahedral |
| 369–480 | π-π* (C=C in benzene) |
Table 2: UV-visible data for the Schiff base ligand and the complexes.
The results of the elemental analysis of the Schiff base and the complexes revealed the actual compositions of the newly synthesised compounds. The experimental values obtained correspond to the calculated compositions of the constituent atoms present in each proposed compound, as shown in Table 3 below.
Products |
% of elements found (calculated) |
Formula |
||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | Fe | Co | Ni | ||
| SBL | 52.35 (52.79) | 5.12 (4.63) | 17. 73 (16.90) | 24.8 (24.01) | - | - | - | C19H18N2O4 |
| SBL-Fe2+ | 39.34 (39.72) | 4.18 (3.96) | 23.03 (22.89) | 3.37 (2.65) | 26.88 (26.14) | - | - | C38H36N4O8Fe |
| SBL-Co2+ | 47.04 (46.31) | 9.71 (8.99) | 10.36 (10.20) | 12.01 (11.98) | - | 20.88 (20.09) | - | C38H36N4O8Co |
| SBL-Ni2+ | 45.23 (45.18) | 4.74 (45.18) | 25.11 (25.01) | 4.4 (4.85) | - | - | 20.52 (20.90) | C38H36N4O8Ni |
Table 3: Elemental analysis of the ligand and the complexes.
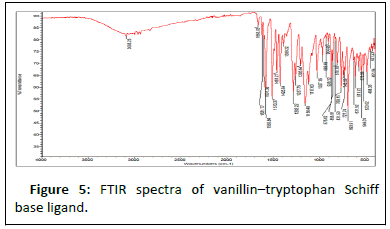
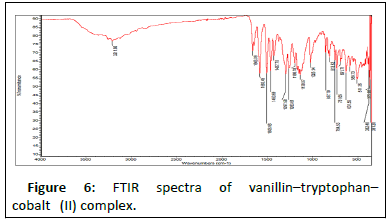
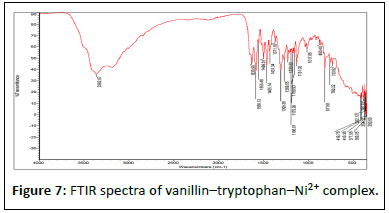
Results in Table 4 below show FTIR spectra bands of the ligand and the complexes at 1628 v (HCN, str), 382.47 v (O-H, str), and 362.12 cm-1, respectively. The FTIR analysis revealed that the bonding of the Schiff base to the metal centers has occurred as expected. When the Schiff base ligand reacted to form complexes with the metal ions, the band shifted to lower frequencies in the 401.64-419.78 cm-1 range for v (MN) and 385.2-535.30 cm-1 range for v (MO), indicating that new bonds had formed and azomethine had been coordinated to the metal ions via oxygen and nitrogen donor atoms [18].
The symmetric carbonyl stretching v (COO-) is also shifted to a higher frequency from 1362 cm-1 to 535.30, 588.73, and 410.36 cm-1 regions for Fe2+, Co2+, and Ni2+ complexes, respectively. Similarly, the v (C–O) phenolic band in the complexes shows a significant shift to lower frequencies of 1282 cm-1 in the ligand and 1026.32, 1025.14, and 1017.85 cm-1 for the metal ions, respectively [19]. The spectra of the complexes show broad bands between 3208.51, 3211.86, and 3362.07 cm-1, which are attributed to O–H stretching vibrations from phenolic groups (Figures 5-10). Information obtained from the results revealed that the Schiff base is a bidentate ligand and that it is bound to the metal ions through the phenolic oxygen and azomethine nitrogen atoms to form octahedral complexes.
| Complexes | V (HC═N) | V (M-N) | V (M-O) | V (COO) | V (C-O) | V (O-H) | V (C-N) | V (C-O-C) | V (C═C) |
|---|---|---|---|---|---|---|---|---|---|
| SBL | 1663 sh | - | - | 1362 sh | 1282 sh | 3326 m | 1373 sh | 1625 m | 1521 sh |
| SBL-Fe2+ | 1583.36 sh | 401.64 sh | 535.30 sh | 535.30 br | 1026.32 sh | 3208.51 br | 1509.46 sh | - | - |
| SBL-Co2+ | 1583.45 sh | 463.69 sh | 511.36 sh | 588.73 sh | 1025.14 | 3211.86 br | 1506.96 sh | - | - |
| SBL-Ni2+ | 1586.13 sh | 419.78 sh | 385.20 sh | 410.36 sh | 1017.85 sh | 3362.07 br | 1630.99 sh | - | - |
Keys: SBL: Vanillin-Tryptophan Schiff Base Ligand; SBL-Fe2+: Vanillin-Tryptophan Schiff Base Fe2+ complex; SBL Co2+: Vanillin-Tryptophan Schiff Base Co2+ complex; SBL-Ni2+: Vanillin-Tryptophan Schiff Base Ni2+ complex. |
|||||||||
Table 4: FT-IR spectral mode (350–4000 cm-1) of the Schiff base and the metal complexes. FT-IR spectral mode (350–4000 cm-1) of the Schiff base and the metal complexes.
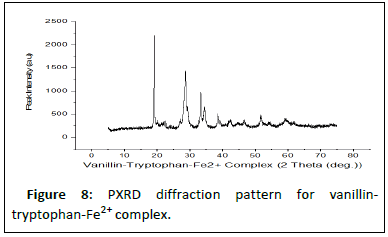
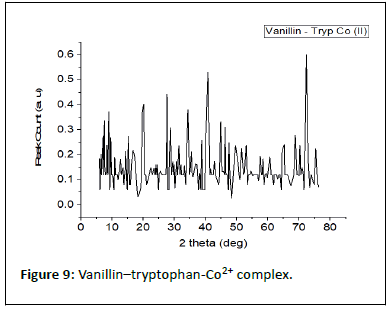
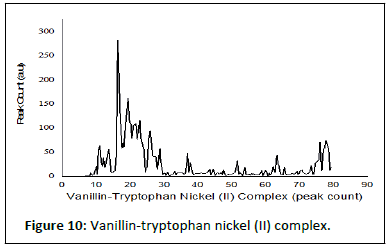
Table 5 shows the results of the PXRD pattern of the complexes scanned at laboratory temperature (303 K) with an XRD machine (RIGAKU). The complexes degree of crystalinity, crystallite sizes, and average crystallite size were measured between 10 and 120° at the wavelength of CuK1 radiation=1.5418 nm. The XRD results are further supported by the interplanar distance and the crystallite sizes (d) of 19.41, 21.32, and 22.16, which correspond to Fe2+-SL, Co2+-SL, and Ni2+-SL, respectively.
| Complexes | Peak position 2θ (o) | FWHM β (o) | % Crystallinity Index (CI) | Crystallite size D (nm) | Average D (nm) |
|---|---|---|---|---|---|
| Fe2+-SL | 53.4 | 0.654 | 75 | 19.41 | 21.63 |
| Co2+-SL | 58.62 | 0.678 | 80 | 21.32 | |
| Ni2+-SL | 62.45 | 0.545 | 84 | 22.16 | |
K=0.94; λ=0.5418 (nm) |
|||||
Table 5: Powder X-rays diffractions analysis.
Table 6 shows the sensitivity test results of the products against some selected pathogens used for the study. The Schiff base is mildly sensitive to one gram +ve and two gram -ve bacterial and fungal spp, the Fe2+ complex is very sensitive to Klebsiella P., Shigella, and a fungi sp C. albican., and the Co2+ complex is effective against Streptococcus P., Klebsiella P., E. coli and A. niger. While the Ni2+ complex proved to be very effective and positive in sensitivity tests for Streptococcus, Klebsiella, E. coli and the fungal species Aspergillus niger.
| S/No | Products | MRSA | S. pnemoniae | ESBL K. pneumoniae | ESBL E. coli | F. shigella | S. typi | C. alicans | A. nigger |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SBL | R | R | MS | MS | MS | R | MS | MS |
| 2 | SBL-Fe2+ | R | R | S | R | S | R | S | R |
| 3 | SBL-Co2+ | MS | S | S | S | R | R | MS | S |
| 4 | SBL-Ni2+ | MS | S | S | S | R | MS | R | S |
| 6 | Standard1* | - | - | - | MS | - | MS | MS | - |
| 7 | Standard2* | - | - | - | - | - | - | MS | - |
| 8 | Control | - | - | - | - | - | - | - | - |
Keys: S: Sensitive; MS: Mild Sensitive; R: Resistance; -: Negative (no reaction); C: Control; Standard1*: Augmentin; Standard2*: Terbinafine |
|||||||||
Table 6: Anti-microbial and antifungal sensitivity test for the Schiff base and complexes.
The data in Table 7 below shows results for the zone of inhibition tests of the products against some of those pathogens that showed positive sensitivity (MIC) tests in Table 5. It also indicates the regions (in mm) where those pathogens are killed or their growth is inhibited by the compounds, with the Schiff base having the smallest inhibition zone (5 mm, 7 mm, 10 mm, 8 mm and 7 mm), followed by the Fe (II) complex (12, 22 and 25 mm), Ni2+ (13, 16, 21 and 23 mm), and the Co2+ complex having the largest inhibition zone (10, 18, 20, 23, and 26 mm, respectively) [20]. The negative test result for the control agar plate is less than 0.01 mm. This indicates that all the microbes grow freely in the absence of the compounds.
| S/No | Compound | MRSA | S. pnemoniae | E. klebsiella pneumoniae | ESBL E. coli | Shigella spp | Salmonella spp | C. albicans | A. nigger |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SBL | - | - | 5 ± 0.2 | 7 ± 0.2 | 10 ± 0.4 | - | 8 ± 0.3 | 7 ± 0.1 |
| 2 | SBL-Fe2+ | - | - | 22 ± 0.1 | - | 25 ± 0.3 | - | 12 ± 0.2 | - |
| 3 | SBL-Co2+ | 20 | 23 ± 0.5 | 26 | 18 | - | - | 20 ± 0.2 | 10 ± 0.5 |
| 4 | SBL-Ni2+ | 21 ± 0.5 | - | - | 23 ± 0.5 | - | 13 | 23 ± 0.2 | 16 ± 0.4 |
| 5 | Control | - | - | - | - | - | - | - | - |
| 6 | Standard1* | 11 | - | 15 | 13 | - | 17 | - | - |
| 7 | Standard2* | - | - | - | - | - | - | 8.5 | 10 |
Key: S: Sensitive; MS: Mild Sensitive; R: Resistance; -: Negative (no reaction); C: Control; Standard1*: Augmentin; Standard2*: Tarbinafin |
|||||||||
Table 7: Shows Zone of Inhibition (ZI) test results of the Schiff base ligands and their metal (II) complexes in (mm).
Table 8 shows the MBC and MFC results of the test compounds, based on the preliminary in vitro antimicrobial activities of the ligand and the complexes. They were screened for their minimum inhibitory concentrations and in vitro antimicrobial actions against the selected microbes [21]. The minimum active concentrations were measured in g/mL, and two standards were used (Augmentin and Terbinafine for fungal spp.). The results revealed that the complexes were more significantly active for killing the pathogens than the ligand, and the overall activities of all three complexes were found to be very effective, even at very low concentrations of less than 250 g/mL, which is being considered to be the minimum concentrations of the Schiff base and complexes required to kill (99.99%) of the pathogens completely [22].
| S/No | Products | MRSA | S. pnemoniae | ESBL K. pneumoniae | ESBL E. coli | F. shigella | S. typi | C. albicans | A. nigger |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SBL | ++ | +++ | -- | - | -- | +++ | -- | -- |
| 2 | SBL-Fe2+ | ++ | ++ | --- | ++ | --- | +++ | -- | + |
| 3 | SBL-Co2+ | --- | -- | -- | - | + | + | --- | -- |
| 4 | SBL-Ni2+ | --- | ++ | + | -- | ++ | -- | -- | -- |
| 5 | Standard1* (mg/mL) | +++ | ---- | ---- | ---- | +++ | ---- | + | +++ |
| 6 | Standard2* | + | +++ | + | +++ | + | ++ | ---- | + |
| (mg/mL) | |||||||||
| 7 | C | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ |
Keys: ++++: very intense microbial growth; +++: high microbial growth (100 -250 mg/ml); ++: moderate microbial growth; ++: mild microbial growth; -: No growth slightly clear at (215 µg/ml); --: No growth very clear at (250 µg/ml); ---: No growth; very –very clear at (500 µg/ml); ----: No growth immediate sharp clear at (1000 µg/ml); C: control; Standard1*: Augmentin; Standard2*: Terbinafine |
|||||||||
Table 8: Shows results of Minimum Bactericidal (MBC) and Fungicidal Concentrations (MFC) (µg/mL) for the Schiff base, metal complexes and standard drugs against the Pathogens used for the study.
Conclusion
Novel Schiff base and new metal (II) complexes has been synthesised, spectroscopic studies has been used to characterized the structure of the Schiff base ligand coordinates with metal ions through the deprotonated hydroxyl, a carbonyl oxygen and nitrogen of the azomethine group. Geometries of the complexes shows that they are all octahedral and the Schiff base ligand is bidentate. The compounds were subjected to test for antimicrobial activities against some infectious pathogen and found to be significantly effective compared to the commonly used antibiotics.
References
- Abu Bakar SN, Bahron H, Kassim K (2010) Synthesis and characterization of a novel Schiff base derived from 2,4,6-trimethyl-m-phenylenediamine with o-vanillin and its metal complexes. 2010 International Conference on Science and Social Research (CSSR 2010), Kuala Lumpur, Malaysia, 463-466.
- Agbese SA, Shallangwa GA, Idris SO (2018) Synthesis, characterization and antimicrobial evaluation of Co (II), Ni (II) and Cu (II) schiff base complexes of (Z)-4-(1-pyridin-4-ylimino) propyl) phenol. Trop J Nat Prod Res (TJNPR) 2:429-432.
- Akhter S, Zaman HU, Mir S, Dar AM, Shrivastava S (2017) Synthesis of Schiff base metal complexes: A concise review. Eur Chem Bull 6:475-483.
- Al-jeboori FHA, Al-shimiesawi TAM (2021) Synthesis of Cr (iii)-aspartate and Cu (ii)-aspartate complexes as antidiabetic compound. Indonesian J Pharm 32:539-547.
- Gupta AK, Orthaber A (2018) Alkynyl coinage metal clusters and complexes syntheses, structures, and strategies. Chemistry 24:7536-7559.
[Crossref] [Google Scholar] [PubMed]
- Barbosa VT, de Menezes JB, Santos JC, de Assis Bastos ML, de Araujo-Junior JX, et al. (2019) Characterization and stability of the antimony-quercetin complex. Adv Pharm Bull 9:432-438.
[Crossref] [Google Scholar] [PubMed]
- Ostovan MA, Khanian MS, Hamidi S, Fattahi M, Dehghani P (2016) Spontaneous multi focal coronary artery spasm: A case report. J Cardiovasc Thorac Res 8:137-139.
[Google Scholar] [PubMed]
- Dhanaraj CJ, Johnson J (2014) Synthesis, characterization, electrochemical and biological studies on some metal (II) Schiff base complexes containing quinoxaline moiety. Spectrochim Acta A Mol Biomol Spectrosc 118:624-631.
[Crossref] [Google Scholar] [PubMed]
- Fugu MB, Ndahi NP, Paul BB, Mustapha AN (2013) Synthesis, characterization, and antimicrobial studies of some vanillin Schiff base metal (II) complexes. J Chem Pharm Res 5:22-28.
- Bahron H, Khaidir SS, Tajuddin AM, Ramasamy K, Yamin BM (2019) Synthesis, characterization and anticancer activity of mono-and dinuclear Ni (II) and Co (II) complexes of a Schiff base derived from o-vanillin. Polyhedron 161:84-92
- Hossain MS, Zakaria CM, Kudrat-E-Zahan M (2018) Metal complexes as potential antimicrobial agent: a review. J Heterocycl Chem 4:1-21.
- Kaczmarek MT, Jastrzab R, Holderna-Kedzia E, Radecka-Paryzek W (2009) Self-assembled synthesis, characterization and antimicrobial activity of zinc (II) salicylaldimine complexes. Inorganica Chimica Acta 362:3127-3133.
- Maalik A, Khan FA, Mumtaz A, Mehmood A, Azhar S, et al. (2014) Pharmacological applications of quercetin and its derivatives: a short review. Trop J Pharm Res 13:1561-1566.
- Suresh MS, Prakash V (2010) Preparation and characterization of Cr (III), Mn (II), Co (III), Ni (II), Cu (II), Zn (II) and Cd (II) chelates of Schiff's base derived from vanillin and 4-aminoantipyrine. Int Phys Sci 5:2203-2211.
- Nagesh GY, Mruthyunjayaswamy BH (2015) Synthesis, characterization and biological relevance of some metal (II) complexes with Oxygen, Nitrogen and Oxygen (ONO) donor Schiff base ligand derived from thiazide and 2-hydroxy-1-naphthaldehyde. J Mol Struct 1085:198-206.
- Neacsu VA, Maxim C, Madalan AM, Hillebrand M, Gonzalez-Arellano C, et al. (2018) New complexes of Ni (II) and Co (III) with a Schiff-base ligand derived from O-vanillin. Crystal structure, magnetic and catalytic properties of a dissymmetric binuclear nickel (II) complex. Polyhedron 150:77-82.
- Sani S, Hussain SY (2017) A convenient method to synthesis and characterization of Ni (ii) and Zn (ii) Schiff base complexes. Int J Innov Res Dev 6:1-5.
- Kumar DP, Jayanthi M, Saranraj P, Karunya SK (2015) Effect of potassium sorbate on the inhibition of growth of fungi isolated from spoiled Bakery products. Life Sci Arch 1:217-222.
- Sheyin FT, Ndukwe GI, Iyun OR, Anyam JV, Habila JD (2018) Phytochemical and anti-microbial screening of crude extracts of natal fig (FicusNatalensisKraus). J Appl Sci Environ Manag 22:1457-1460.
- Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, et al. (2010) IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol 185:5953-5961.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang X, Ding L (2010) Interaction between tryptophan-vanillin Schiff base and herring sperm DNA. J Serb Chem Soc 75:1191-1201.
- Zurowska B, Boduszek B (2011) Synthesis, spectroscopy and magnetic properties of transition-metal complexes with aminophosphonate derivatives of pyridine. Mater Sci-Poland 29:105-111.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences