An Investigation of Enzymatic Phosphorolysis of ÃÆà ½Ãâñ(1ÃÆâÃâââ¬Â Ãâââ¬â¢4)-Linked Oligo-d-glucosaminides by Thermostable ÃÆà ½Ãâñ-Glucan Phosphorylase Catalysis
Jun-ichi Kadokawa, Kento Yamashita, Riko Shimohigoshi and Kazuya Yamamoto
DOI10.21767/2472-1123.100010
Jun-ichi Kadokawa*, Kento Yamashita, Riko Shimohigoshi and Kazuya Yamamoto
Graduate School of Science and Engineering, Kagoshima University, 1-21-40 Korimoto, Kagoshima 890-0065, Japan
- *Corresponding Author:
- Jun-ichi Kadokawa
Graduate School of Science and Engineering
Kagoshima University
1-21-40 Korimoto
Kagoshima 890-0065, Japan
Tel: + 81992857743
E-mail: kadokawa@eng.kagoshima-u.ac.jp
Received date: May 11, 2016; Accepted date: June 22, 2016; Published date: June 27, 2016
Citation: Kadokawa J, Yamashita K, Shimohigoshi R, et al. An Investigation of Enzymatic Phosphorolysis of α(1→4)-Linked Oligo-D-glucosaminides by Thermostable α-Glucan Phosphorylase Catalysis. J Org Inorg Chem. 2016, 2:1.
Abstract
This paper reports an investigation of enzymatic phosphorolysis of α(1→4)- linked oligo-d-glucosaminide substrates by thermo stable α-glucan phosphorylase (from Aquifex aeolicus VF5) catalysis. α-Glucan phosphorylase catalyzes both phosphorolysis and glucosylation/polymerization at the nonreducing end of α(1→4)-linked glucose substrates depending on conditions. The authors also found that α(1→4)-linked d-glucosaminide polymer (chitosan stereoisomer) was obtained by the thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of α-d-glucosamine 1-phosphate from a maltooligosaccharide primer. In the present study, we intend to reveal whether the enzyme catalyzes phosphorolysis of substrates containing such d-glucosaminide chains. The α(1→4)-linked oligo-d-glucosaminide substrates elongated from maltotriose were first prepared by the thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of α-d-glucosamine 1-phosphate from the maltotriose primer and subsequent purification by preparative HPLC. The phosphorolysis of the resulting substrates are conducted in the presence of thermostable α-glucan phosphorylase in phosphate buffer. The analytical results of the products fully supported the occurrence of phosphorolysis at the nonreducing end of both the chains of d-glucosamine-α(1→4)-d-glucosamine and d-glucosamine-α(1→4)-d-glucose sequences.
Keywords
α-glucan phosphorylase; α(1→4)-linked D-glucosaminides; Phosphorolysis
Introduction
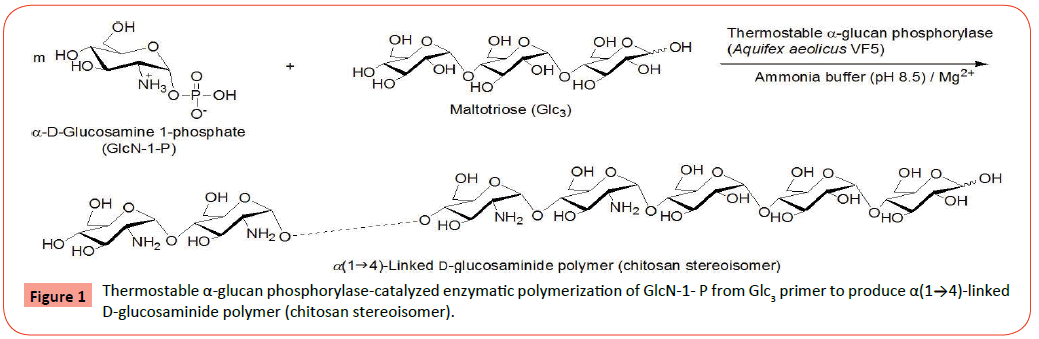
Enzymatic polymerization has been identified as a powerful approach to synthesize polymerizationcomposed of stereo- and regio-controlled glycosidic linkages [1-6]. α-Glucan phosphorylase is one of the enzymes that have practically been used as a catalyst for enzymatic polymerization to produce well-defined polysaccharides. α-Glucan phosphorylase catalyzes in vivo phosphorolysis of α(1→4)-glucans such as amylose and glycogen at the nonreducing end in the presence of inorganic phosphate (Pi) to produce α-D-glucose 1-phosphate (Glc-1-P) [7-9]. Because of the reversibility of such phosphorolytic reaction, this enzyme also catalyzes in vitro glucosylation of α(1→4)-glucan as a glycosyl acceptor such as maltooligosaccharide using Glc-1-P as a glycosyl donor to produce α(1→4)-glucosidic linkage with liberating Pi [10]. The reversible reaction by α-glucan phosphorylase catalysis is summarized as follows; [α (1→4)-Glc]n+1+Pi → [α (1→4)-Glc] n+Glc-1-P. Under the conditions in the large donor/acceptor ratios, successive glucosylations at the elongating nonreducing end from maltooligosaccharide as a primer take place by α-glucan phosphorylase catalysis, leading to the enzymatic polymerization to produce (1→4)-linked polysaccharide, that is, amylose [11-13]. The degree of polymerization (DP) of amylose can be controlled by the donor/acceptor feed ratio.
α-Glucan phosphorylases have exhibited the different specificity for the recognition of substrates, depending on their sources. For example, the smallest substrates recognized by the most common α-glucan phosphorylase isolated from potato (potato α-glucan phosphorylase) in phosphorolysis and glucosylation reactions are typically maltopentaose (Glc5) and maltotetraose (Glc4), respectively. On the other hand, Glc4 and maltotriose (Glc3) are the smallest substrates recognized by α-glucan phosphorylase isolated from thermophilic bacterial sources (thermostable α-glucan phosphorylase) in phosphorolysis and glucosylation reactions, respectively [13-16]. Moreover, α-glucan phosphorylases are known to show weak specificity for the recognition of substrates, which has also been found to be depended on their sources [17-19]. Potato α-glucan phosphorylase catalyzes enzymatic glucosaminylations using α-D-glucosamine 1-phosphate (GlcN- 1-P) and its derivative, N-formyl-α-D-glucosamine 1-phosphate (GlcNF-1-P), as non-native glycosyl donors in acetate buffer to produce oligosaccharides having one GlcN(F) unit at the nonreducing end [20,21], whereas successive glucosaminylations using GlcN-1-P occur by thermostable α-glucan phosphorylase (from Aquifex aeolicus VF5) catalysis in acetate buffer, giving rise to α(1→4)-linked oligo-GlcN chains at the nonreducing end of glycosyl acceptors, e.g., Glc3, the smallest glycosyl acceptor for this enzyme. With increasing the donor/acceptor feed ratio, however, the DP values of the α(1→4)-GlcN chains retained at most five. This result indicated that further chain-elongation was inhibited by Pi produced from GlcN-1-P, because which is a native substrate for phosphorolysis by α-glucan phosphorylase catalysis. An attempt was then made to remove Pi as a precipitate from the enzymatic glucosaminylation field by performing the reaction in an ammonium buffer (0.5 M, pH 8.5) containing MgCl2, because the previous publication has reported that Pi forms an insoluble salt with ammonium and magnesium ions [22]. Consequently, the successive enzymatic glucosaminylations were accelerated in the buffer system to occur polymerization of GlcN-1-P from the Glc3 primer, giving rise to the α(1→4)-linked D-glucosaminide polymer, which corresponded to the structure of a chitosan stereoisomer (Figure 1) [23,24]. On the basis of the above results, we have speculated that thermostable α-glucan phosphorylase catalyzes not only the glucosaminylation using GlcN-1-P, but also the phosphorolysis at the nonreducing end of the α(1→4)- linked glucosaminide chain, depending on the concentration of Pi. Moreover, it was reported that thermostable α-glucan phosphorylase (from Escherichia coli) exhibited the different catalytic behaviors toward phosphorolysis and glucosylation depending on reaction conditions [15]. In the present paper, we report the precise investigation to reveal whether thermostable α-glucan phosphorylase catalyzes phosphorolysis of α(1→4)- linked oligo-D-glucosaminides-maltotriose (GlcNm-Glc3) substrates.
Experimental
Materials
A substrate, GlcN-1-P, was synthesized according to previously reported procedure [25]. Thermostable α-glucan phosphorylase from Aquifex aeolicus VF5 [26] was kindly supplied by Ezaki Glico Co. Ltd, Osaka, Japan. Other reagents and solvents were used as commercially received.
Synthesis of substrates for phosphorolysis, GlcNm-Glc3
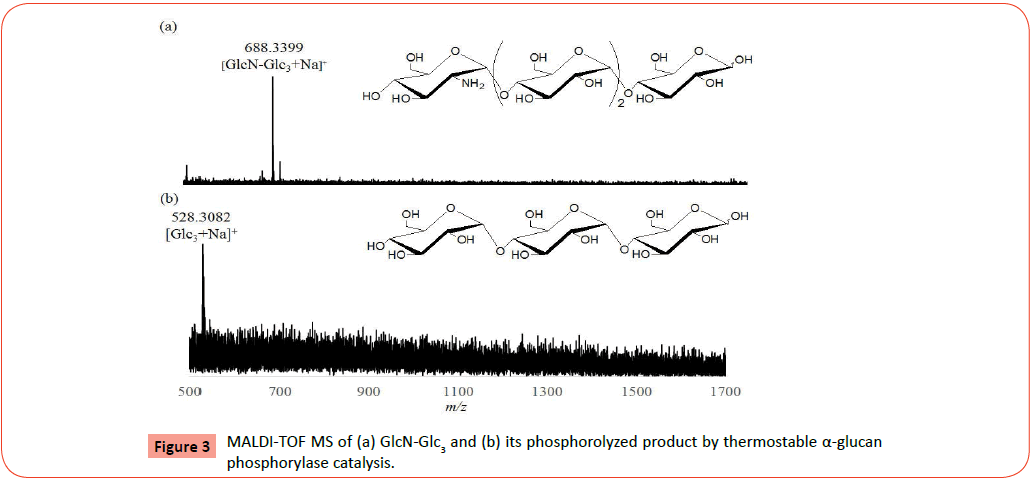
A mixture of GlcN-1-P (0.1145 g, 0.44 mmol) and Glc3 (0.0222 g, 0.044 mmol) in 0.1 M ammonia buffer (pH 8.5, 3.0 mL) dissolving MgCl2 was incubated in the presence of thermostable α-glucan phosphorylase (21 U) at 40°C for 48 hrs. After the reaction mixture was maintained at 100 °C for 1 h in order to deactivate the enzyme, the precipitate was removed by centrifugation and the supernatant was lyophilized. Glucoamylase (GA, 20 U) was added to a solution of the resulting product in water (1.0 mL) and the mixture was incubated at 40°C for 1 h. The reaction mixture was then maintained at 100°C for 1 h in order to deactivate the enzyme. The deactivated enzyme was removed by centrifugation and the supernatant was lyophilized and subjected to MALDI-TOF measurement. To a dispersion of the resulting product (0.1658 g) in acetone (5.0 mL), 0.5 M methanolic hydrochloric acid (1.0 mL) was added dropwise and the mixture was stirred at room temperature for 15 min. The filtration of the dispersion gave the hydrochloride salt of the product. The resulting crude product (0.1650 g) was dissolved in water (1.0 mL) and subjected to preparative HPLC with Wakosil 5NH2 column (10 × 250 mm) with acetonitrile/water (3:1, v/v) as the eluent (flow rate; 2.0 mL/min) to isolate a tetrasaccharide (GlcN-Glc3) and a mixture of pentaand hexasaccharides (GlcN2-Glc3, GlcN3-Glc3).
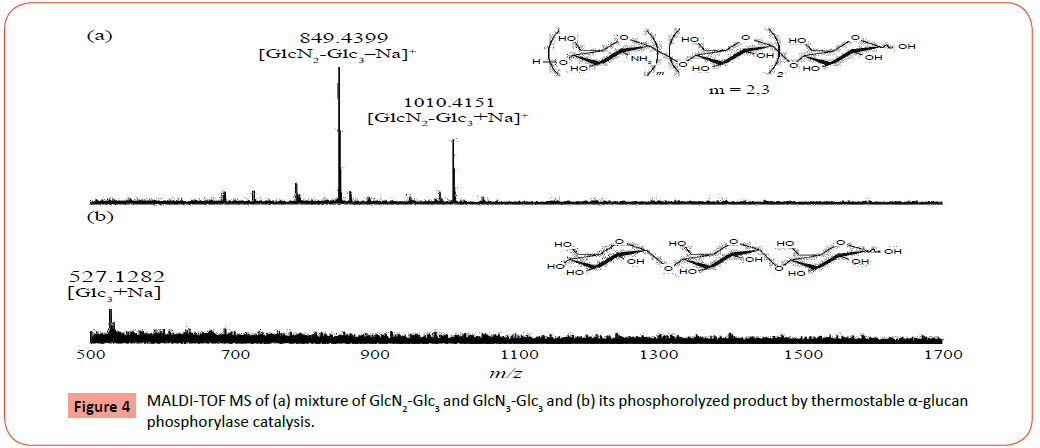
MALDI-TOF MS: [GlcN2-Glc3+Na]+: Calcd m/z 688.5511, Found m/z 688.3399, [GlcN2-Glc3+Na]+, [GlcN3-Glc3+Na]+: Calcd m/z 849.5557, 1010.5670, Found m/z 849.4399, 1010.4151.
Phosphorolysis of GlcN-Glc3
The isolated GlcN-Glc3 (1.7 mg) was treated with aqueous NaHCO3 (0.22 mg, 2.62 mmol) at room temperature for 15 min for dehydrochlorination. After the resulting solution was diluted with 0.2 M phosphate buffer (pH 6.2, 2.0 mL), the mixture was incubated in the presence of thermostable α-glucan phosphorylase (21 U) at 40°C for 2 days. After the reaction mixture was maintained at 100 °C for 1 h in order to deactivate the enzyme, the precipitate was removed by centrifugation and the supernatant was lyophilized to give the phosphorolyzed product.
MALDI-TOF MS: [Glc3+Na]+: Calcd m/z 527.4268, Found m/z 528.3082
Phosphorolysis of mixture of GlcN2-Glc3 and GlcN3-Glc3
The mixture of GlcN2-Glc3 and GlcN3-Glc3 (1.3 mg) was treated with aqueous NaHCO3 (0.14 mg, 1.66 mmol) at room temperature for 15 min for dehydrochlorination. After the resulting solution was diluted with 0.2 M phosphate buffer (pH 6.2, 2.0 mL), the mixture was incubated in the presence of thermostable α-glucan phosphorylase (21 U) at 40 °C for 2 days. After the reaction mixture was maintained at 100 °C for 1 h in order to deactivate the enzyme, the precipitate was removed by centrifugation and the supernatant was lyophilized to give the phosphorolyzed product.
MALDI-TOF MS: [Glc3+Na]+: Calcd m/z 527.4268, Found m/z 527.1282
Measurements
1H NMR spectra were recorded on JEOL ECA 600 and ECX 400 spectrometers. MALDI-TOF MS measurements were conducted by using SHIMADZU Voyager Biospectrometry Workstation Ver. 5.1 with 2,5-dihydroxybenzoic acid as a matrix containing 0.05% trifluoroacetic acid under positive ion mode. Analytical HPLD was performed on a PU-2080 apparatus (JASCO) using RI-2031 RI detector (JASCO) with MERCK LiChrosher 100 NH2 column (10 mm, 4.0 × 250 mm) with acetonitrile/water (3:2, v/v) as the eluent (flow rate; 0.5 mL/min). Preparative HPLC was performed on a NPL apparatus (Nihon Seimitsu Kagaku Co., LTD) using YRD- 833 RI detector (Shimamuratech) with Wakosil 5NH2 column (10 × 250 mm) with acetonitrile/water (3:1, v/v) as the eluent (flow rate; 2.0 mL/min).
Results and Discussion
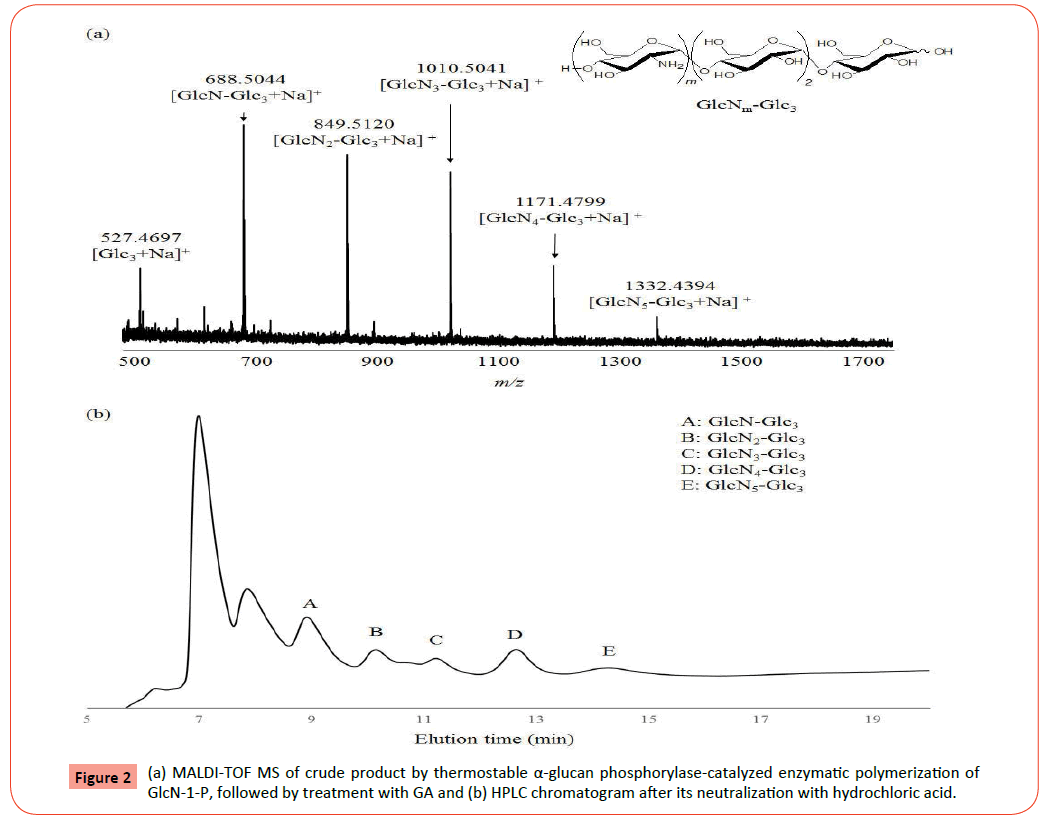
We first prepared the α(1→4)-linked oligo-D-glucosaminidesmaltotriose substrates for the phosphorolysis, that is, tetrahexasaccharides (GlcN-Glc3, GlcN2-Glc3, and GlcN3-Glc3) by the thermostable phosphorylase-catalyzed enzymatic polymerization of GlcN-1-P according to the literature procedure (Figure 1) [23]. The smallest substrate, Glc3, was used as the primer for the polymerization to avoid the occurrence of phosphorolysis of the primer, because the enzyme does not catalyze its phophorolysis. The reaction was performed in the GlcN-1-P/Glc3 feed ratio of 10:1 in the presence of thermostable α-glucan phosphorylase in ammonia buffer (pH 8.5) containing Mg2+ ion at 40°C for 48 h. The shorter reaction time was employed in this study than the literature condition (7 days) to produce GlcNm-Glc3 with small DP values. The MALDI-TOF MS of the crude product after GAtreatment observes several peaks corresponding to the molecular masses of oligosaccharides containing one-five GlcN units (Figures 2a and 2b), supporting the production of the shorter GlcNm-Glc3 than the literature products (m=~ 12). Moreover, the MALDI-TOF MS result also indicates no occurrence of the exo-type hydrolysis at the Glc3 segment in the produced oligosaccharides by GA catalysis, suggesting the presence of the GlcN chains at the nonreducing end of Glc3. The HPLC chromatogram after neutralization of the product with hydrochloric acid shows the plural peaks corresponding to oligosaccharides with different DPs. Accordingly, the fractions of a peak A corresponding to a tetrasaccharide and peaks B and C corresponding to pentaand hexasaccharides were collected by preparative HPLC. The MALDI-TOF MS of the former and latter fractions shows peaks corresponding to molecular masses of GlcN-Glc3 and GlcN2-Glc3/ GlcN3-Glc3, respectively (Figures 3a and 4a), indicating that the tetrasaccharide and a mixture of the penta- and hexasaccharides were successfully isolated.
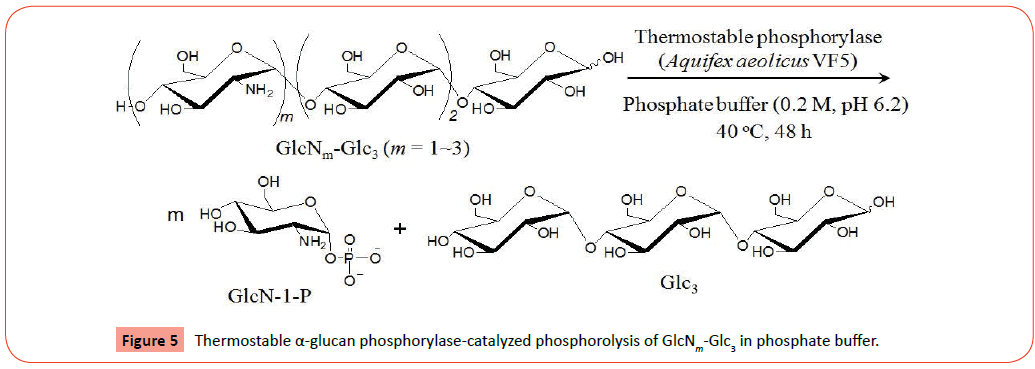
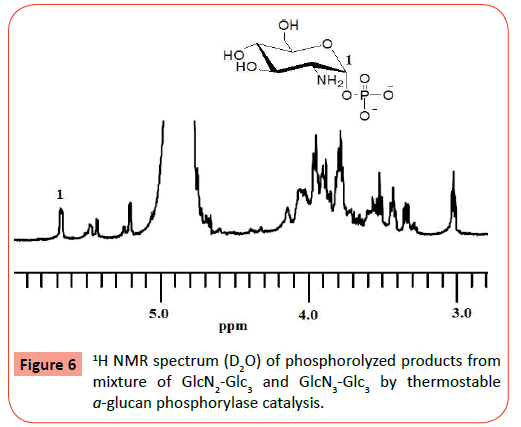
The obtained two samples were then subjected to the thermostable α-glucan phosphorylase-catalyzed phosphorolysis reaction (Figure 5). The tetrasaccharide, GlcN-Glc3, after dehydrochlorination by NaHCO3 was incubated in the presence of thermostable α-glucan phosphorylase in phosphate buffer (pH 6.2, source of Pi) at 40 °C for 48 h. After deactivation of the enzyme, the reaction mixture was lyophilized and subjected to the MALDI-TOF MS measurement. The MALDI-TOF MS of the product (Figure 3b) observes a peak corresponding to the molecular mass of Glc3, but does not show the molecular mass peak of GlcN-Glc3. In the MALDI-TOF MS of the product (Figure 4b) by the same procedure using the GlcN2-Glc3/GlcN3-Glc3 sample, a molecular mass peak of Glc3 is also detected, but the molecular mass peaks of GlcN2-Glc3/GlcN3-Glc3 are not observed. These results strongly suggested the occurrence of the thermostable α-glucan phosphorylase-catalyzed phosphorolysis of all the substrates, GlcN-Glc3, GlcN2-Glc3, and GlcN3-Glc3 in the presence of Pi. Both the 1H NMR spectra (D2O) of the products by the above two reactions indicated the production of GlcN-1-P, which also supported the occurrence of the phosphorolysis by thermostable α-glucan phosphorylase catalysis. For example, the 1H NMR spectrum (D2O) of the products from the GlcN2-Glc3/GlcN3-Glc3 sample (Figure 6) observes the characteristic signal due to H-1 proton of GlcN-1-P at around 5.7 ppm (multiplet). All the above results revealed that thermostable α-glucan phosphorylase catalyzed phosphorolysis at the nonreducing ends of both the chains of GlcN-α(1→4)-GlcN and GlcN-α(1→4)-Glc sequences in the presence of Pi.
Conclusion
In this paper, we revealed that thermostable α-glucan phosphorylase from Aquifex aeolicus VF5 catalyzed phosphorolysis at the nonreducing ends of both the chains of GlcN-α(1→4)- GlcN and GlcN-α(1→4)-Glc sequences in the presence of Pi. The substrates, GlcNm-Glc3 (m=1~3), were first prepared by the thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of GlcN-1-P from the Glc3 primer in ammonia buffer containing Mg2+ ion and isolated by preparative HPLC. The thermostable α-glucan phosphorylase-catalyzed phosphorolysis of the resulting substrates was then conducted in phosphate buffer. The MALDI-TOF MS of the products suggested that the enzymatic phosphorolysis of the substrates were fully progressed to produce Glc3. The production of GlcN-1-P was observed by the 1H NMR spectra of the products, which also supported the progress of phosphorolysis. The findings in the present study will contribute to further development of the precise enzymatic reactions by α-glucan phosphorylase catalysis to produce welldefined saccharide materials.
Acknowledgements
This work was financially supported by a Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science, and Technology, Japan (No. 25620177). We acknowledge the supplement of thermostable α-glucan phosphorylase from Ezaki Glico Co. Ltd., Osaka, Japan.
References
- Kobayashi S, Uyama H, Kimura S (2001) Enzymatic polymerization. Chem Rev 101: 3793-3818.
- Shoda S, Izumi R, Fujita M (2003) Green process in glycotechnology. Bull Chem Soc Jpn 76: 1-13.
- Kobayashi S, Makino A (2009) Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem Rev 109:5288-5353.
- Kadokawa J, Kobayashi S (2010) Polymer synthesis by enzymatic catalysis. Curr Opin Chem Biol 14:145-153.
- Kadokawa J (2011) Precision polysaccharide synthesis catalyzed by enzymes. Chem Rev 111: 4308-4345.
- Shoda S, Uyama H, Kadokawa J, Kimura S, Kobayashi S (2016) Enzymes as green catalysts for precision macromolecular synthesis. Chem Rev 116: 2307-2413.
- Kitaoka M, Hayashi K (2002) Carbohydrate-processing phosphorolytic enzymes. Trends Glycosci Glyc 14: 35-50.
- Nakai H, Kitaoka M, Svensson B, Ohtsubo K (2013) Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr Opin Chem Biol 17: 301-309.
- Puchart V (2015) Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol Adv 33: 261-276.
- Seibel J, Jordening HJ, Buchholz K (2006) Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal Biotransfor 24:311-342.
- Ziegast G, Pfannemüller B (1987) Phosphorolytic syntheses with di-, oligo-and multi-functional primers. Carbohydrate research 160: 185-204.
- Fujii K, Takata H, Yanase M, Terada Y, Ohdan K, et al. (2003) Bioengineering and application of novel glucose polymers. Biocatal Biotransfor 21: 167-172.
- Yanase M, Takaha T, Kuriki T (2006) α-Glucan phosphorylase and its use in carbohydrate engineering. J Sci Food Agr 86:1631-1635.
- Boeck B, Schinzel R (1996) Purification and characterisation of an α-glucan phosphorylase from the thermophilic bacterium Thermus thermophilus. Eur J Biochem 239:150-155.
- Takaha T, Yanase M, Takata H, Okada S (2001) Structure and properties of Thermus aquaticusα-glucan phosphorylase expressed in Escherichichia coli. J Appl Glycosci 48:71-78.
- Yanase M, Takata H, Fujii K, Takaha T, Kuriki T (2005) Cumulative effect of amino acid replacements results in enhanced thermostability of potato type L a-glucan phosphorylase. Appl Environ Microbiol 71:5433-5439.
- Kadokawa J (2011) Facile synthesis of unnatural oligosaccharides by phosphorylase-catalyzed enzymatic glycosylations using new glycosyl donors. Nova Science Publishers USA,pp: 269-281.
- Kadokawa J (2013) Synthesis of non-natural oligosaccharides by α-glucan phosphorylase-catalyzed enzymatic glycosylations using analogue substrates of α-D-glucose 1-phosphate. Trends Glycosci Glyc 25:57-69.
- Kadokawa J (2015) Enzymatic synthesis of non-natural oligo- and polysaccharides by phosphorylase-catalyzed glycosylations using analogue substrates. In: Cheng HN, Gross RA,Smith PB (eds) Green Polymer Chemistry: Biobased Materials and Biocatalysis, ACS Symposium Series; American Chemical Society, USA 1192: 87-99.
- Nawaji M, Izawa H, Kaneko Y, Kadokawa J (2008) Enzymatic a-glucosaminylation of maltooligosaccharides catalyzed by phosphorylase. Carbohyd Res 343:2692-2696.
- Kawazoe S, Izawa H, Nawaji M, Kaneko Y, Kadokawa J (2010) Phosphorylase-catalyzed Nformyl- α-glucosaminylation of maltooligosaccharides. Carbohyd Res 345:631-636.
- Borgerding J (1972) Phosphate deposits in digestion systems. J Water Pollut Control Fed 44:813-819.
- Kadokawa J, Shimohigoshi R, Yamashita K, Yamamoto K (2015) Synthesis of chitin and chitosan stereoisomers by thermostable a-glucan phosphorylase-catalyzed enzymatic polymerization of α-D-glucosamine 1-phosphate. Org Bimol Chem 13:4336-4343.
- Yamashita K, Yamamoto K, Kadokawa J (2015) Synthesis of non-natural heteroaminopolysaccharides by α-glucan phosphorylase-catalyzed enzymatic copolymerization: α(1→4)-linked glucosaminoglucans. Biomacromolecules 16:3989-3994.
- Takata Y, Shimohigoshi R, Yamamoto K, Kadokawa J (2014) Enzymatic synthesis of dendritic amphoteric a-glucans by thermostable phosphorylase catalysis. Macromol Biosci 14:1437-1443.
- Bhuiyan SH, Rus’d AA, Kitaoka M, Hayashi K (2003) Characterization of a hyperthermostable glycogen phosphorylase from Aquifex aeolicusexpressed in Escherichia coli. Journal of Molecular Catalysis B: Enzymatic 22:173-180.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences