Treatments Discovery so Far on SARS-COVID-19: A Brief Report
Nobendu Mukerjee*, Malay Dolai
Nobendu Mukerjee*, Malay Dolai
1Department of Microbiology, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata, India
2Department of Chemistry ,Prabhat Kumar College ,Contai, Purba Medinipur 721404,West Bengal, India
- *Corresponding Author:
- Nobendu Mukerjee
Department of Microbiology, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata, India
Tel: 07044326745
E-mail:nobendumukerjee00@gmail.com
Received Date: April 04, 2020; Accepted Date: September 06, 2021; Published Date: September 15, 2021
Citation: Mukerjee N (2021) Treatments Discovery so Far on SARS-COVID-19: A Brief Report. J Org Inorg Chem Vol: 7 No: 5.
Abstract
After the attack of Influenza virus (also known as Flu virus) in the year 1918 which after turned to be a pandemic, now the world is again facing a similar situation since March 2020. However, with the advancement in field of medicinal science has made it possible to identify the novel infectious agent from the corona-viridae family. SARS-CoV-2 belongs to the broad family of viruses known as coronaviruses. It is a positive-sense single-stranded RNA virus, with a single linear RNA segment. Other coronaviruses are capable of causing illnesses ranging from the common cold to more severe diseases such as Middle East respiratory syndrome (MERS, fatality rate ~34%). Rapid sequencing by various groups helped in identifying the structure and function of the virus, it’s immunogenicity in diverse populations, and potential preventive measures. As of December 2020 there were over 150+ vaccine candidates for COVID-19 being developed. Out of these, at least 52 candidate vaccines are initiated for trials in human model. There are several others currently in phase 2/3 which would enter at 3rd phase in the few months.
https://casinopluss.com https://vdcasinogirisi.com https://betriyal.info https://betriyal.org https://betriyal.co https://betriyal.xyz https://betriyal.biz https://betriyal.fun https://betriyal.club https://betriyalgiris.com https://betriyal163.com https://casinoplus.club https://casinoplus.fun https://casinoplus.xyz https://maltcasino.xyz https://almanbahise.com https://melbete.com https://betsatgirisi.com https://fenomengiris.com https://betmatik-giris.com
Keywords
Immune response; Placebo, Immunoresponse; Drug repurposing and remodeling; SARS-CoV-2
Introduction
Coronavirus attacks the respiratory system’s receptors because the virus accesses host cells via the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant in the type-II alveolar cells of lungs which also causes pneumonia and lymphopenia in maximum infected individuals. Viral subunits like spike and nucleocapsid proteins trigger an immune response in the host which eliminate the virus. These viral antigens can be either recognized by the B cells or by MHC complexes to the T-cells, resulting in antibody production, increased in cytokine secretion, and cytolytic activity in the acute phase of infection. Genetic polymorphism in MHC enables it to present some of the T-cell surface very well over the other MHC alleles. Studies have reported that infected individuals can, after recovery, induce strong protective responses by generating a memory T-cell pool against SARS-CoV and MERS-CoV. These memory T-cells were not persistent in the long term and, upon reactivation, caused local damage due to cross-reactivity. So far, the reports suggests that SARS-CoV-2 is highly contagious and it shows related symptoms in three different stages and develops an exhaustive T-cell pool at higher loads of viral infection. As there are no specific treatments available for this novel coronavirus, numerous small molecular drugs that are being used for the treatment of diseases like SARS, MERS, HIV, Ebola, Malaria, and Tuberculosis are being given to COVID-19 patients, and clinical trials for many such drugs have already begun. A classical immune-therapy of convalescent plasma transfusion from recovered patients has also been initiated for the neutralization of viremia in terminally effected COVID-19 patients.
Treatment strategies of covid-19
There were more than 150 vaccines under trial for Covid-19 and among them more than 50 were for human model trial. Earlier, the treatment focuses mainly on providing intensive care in order to the symptoms and discomfort associated with COVID-19. Convalescent fluid therapy/ plasma therapy accompanied by broad-spectrum antibiotics were also given to the patients as a protective measure to avoid opportunistic bacterial infections. However, ventilator support for respiration is provided to the patients under extreme conditions of short breathlessness. Numerous FDA screened antiviral drugs, vaccines, and immunological therapies that are already being used to treat other diseases have also been considered as a possible approach for treating COVID-19. But this approach may reduce the availability of drugs and vaccines for the intended diseases and for the patients with the greatest need. The molecular, structural, and functional relationships of SARS-CoV-2 with SARS-CoV might define the use of existing anti-viral drugs against COVID-19, considering the total time it takes to perform clinical trials and get FDA approval for the use of novel drugs and vaccines. The increasing knowledge of the genetic, immunological, and molecular mechanisms behind it’s enhanced pathogenicity might help in developing specific treatment approaches for COVID-19 in the future.
MMR Vaccine Appears to Confer Strong Protection from COVID-19
COVID-19 infections have presented with a very unusual morbidity penetration, where patients younger than 50 show little morbidity from the disease, with mortality dramatically increasing above age 50. This is a very different presentation from other viral diseases, suggesting that some factor is protective in younger people, and missing in older patients. It was our theory that different exposure to vaccines between younger and older people may account for this different morbidity rate. Widely deployed measles-rubella containing vaccines (MRCV) including MMR, MR, and MMRV are believed to be for which children, teenagers and other young adults often have few symptoms from COVID-19, and few deaths are attributed to COVID-19 in the young. Statistical data also demonstrates that MRCV vaccination rates substantially correlate with the widely varying outcomes from country to country related to COVID-19 mortality. Countries with recent, major MRCV vaccination programs have few if any deaths from COVID-19.
Reason why so many vaccines are in development?
Many vaccine candidates will be evaluated before any are found to be both safe and effective. For example, of all the vaccines that are studied in the lab and laboratory animals, roughly 7 out of every 100 will be considered good enough to move into clinical trials in humans. Of the vaccines that do make it to clinical trials, just one in five is successful. Having lots of different vaccines in development increases the chances that there will be one or more successful vaccines that will be shown to be safe and efficacious for the intended prioritized populations.
Various vaccines trailed against COVID-19
It is generally accepted that only vaccines can halt the spread of the pandemic virus; thus, several groups have already published interim results of phase 1/2 clinical trials of SARSCoV- 2 vaccines generated on various vaccine platforms. It is critical to accumulate as many clinical data on the safety and immunogenicity of SARS-CoV-2 vaccines as possible, because this infection is new to the human population and all possible short-term or long-term rare adverse events are difficult to predict. A study of β-propiolactone and inactivated aluminium hydroxide-adjuvant whole virion SARS-CoV-2 vaccine candidate was developed by China National Biotech Group and the Beijing Institute of Biological Products (BBIBP-CorV), which was tested in randomised, placebo-controlled phase 1/2 clinical trials in healthy individuals aged 18 years and older. This was the first study of an inactivated SARS-CoV-2 vaccine to include participants more than 60+. The current study is the report of interim results of safety and immunogenicity of inactivated SARS-CoV-2 vaccine, with the first being the another β- propiolactone inactivated aluminium-adjuvant whole virion SARS-CoV-2 vaccine developed by Wuhan Institute of Biological Products.6 Both studies showed very similar levels of adverse events and neutralising antibody titres post vaccination, which indicates the reproducibility of clinical results of similar vaccine modes produced by different manufacturers. Although the use of whole virion vaccines ensures the presence of all potential immunogenic epitopes, a critical consideration for the safety and efficacy of the vaccines is the structural stability of the major antigenic determinants. As has been shown for other inactivated vaccines, improper inactivation processes can alter the antigenic properties of epitopes, resulting in the induction of non-neutralising antibodies, which can lead to the disease enhancement rather than protection. With this in mind, encouraging results have been obtained when testing BBIBPCorV in various animal models, where no disease enhancement on SARS-CoV-2 challenge was found.9 However, we need to acknowledge that for this new infection, all possible animal models have not yet been worked out for simulating antibodydependent disease enhancement in humans. Xia S et al. 2020.
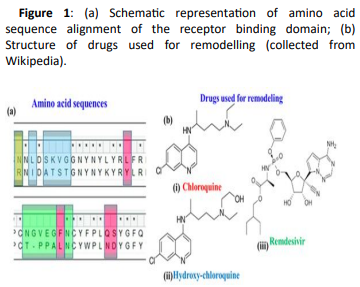
Use of Chloroquine and Hydroxy-Chloroquine derivatives for amino acid docking
The present research aims at investigating the therapeutic potential of Chloroquine and its potent derivative Hydroxychloroquine against SARS-CoV-2 viral proteins. Hydroxychloroquine is an anti-malarial drug that has shown also efficacy in Q-fever and Whipple disease (Thropheryma Whipplei). Hydroxy-chloroquine has also been effectively administered in patients having Systemic Lupus Erythematosus (SLE), rheumatoid arthritis and sarcoidosis with skin manifestations and refractory hypercalciuria. Hydroxy-chloroquine acts through increase of lysosomal pH in antigen presenting cells and as an inhibitor of autophagy. Anti-viral properties were also attributed to a mechanism involving interference with glycosylation of angiotensin converting enzyme 2 (ACE-2), the cellular receptor of SARS-CoV. It shows high tissue absorption with a terminal half-life of almost 40 days that is mainly attributed to high-tissue deposition and not reduced clearance. Its major side-effects are vomiting, headache, changes in vision i.e. retinopathies, muscle weakness and QT prolongation.
Recently, Chloroquine and its derivative Hydroxychloroquine have garnered enormous interest amongst the clinicians and health authorities’ world over as a potential treatment to contain COVID-19 pandemic. At the same time screening was performed for some chemically synthesized derivatives of Chloroquine and compared their binding efficacy with chemically synthesized Chloroquine derivatives through in silico approaches. Some essential viral proteins and enzymes were selected that are implicated in SARS-CoV-2 replication and multiplication as putative drug targets. Chloroquine, Hydroxychloroquine, and some of their chemically synthesized derivatives, taken from earlier published studies were selected as drug molecules. Scientists have conducted molecular docking and related studies between Chloroquine and its derivatives and SARS-CoV-2 viral proteins, and the findings show that both Chloroquine and Hydroxychloroquine can bind to specific structural and non-structural proteins implicated in the pathogenesis of SARS-CoV-2 infection with different efficiencies. Our current study also shows that some of the chemically synthesized Chloroquine derivatives can also potentially inhibit various SARS-CoV-2 viral proteins by binding to them and concomitantly effectively disrupting the active site of these proteins. These findings bring into light another possible mechanism of action of Chloroquine and Hydroxychloroquine and also pave the way for further drug repurposing and remodelling.
Use of Remdesivir
Remdesivir was at first developed for Ebola virus and now the first drug approved by the FDA for treating the SARS-CoV-2 virus. It is indicated for treatment of COVID-19 disease in hospitalized adults and children aged 12 years and older who weigh at least 40 kg. The broad-spectrum antiviral is a nucleotide analogue prodrug. Remdesivir reduces the recovery time of hospitalized patients who require supplemental oxygen and may have a positive impact on mortality outcomes while having a favourable safety profile. Although it’s an important milestone in the fight against COVID-19, approval of this drug will not be sufficient to solve the public health issues caused by the ongoing pandemic.
Use of Astra Zeneca
COVID-19 Vaccine AstraZeneca is expected to work by preparing the body to defend itself against infection with the coronavirus SARS-CoV-2. This virus uses proteins on its outer surface, called spike proteins, to enter the body’s cells and cause disease. COVID-19 Vaccine Astra Zeneca is made up of another virus (of the adenovirus family) that has been modified to contain the gene for making the SARS-CoV-2 spike protein. The adenovirus itself cannot reproduce and does not cause disease. Once it has been given, the vaccine delivers the SARS-CoV-2 gene into cells in the body. The cells will use the gene to produce the spike protein. The person’s immune system will treat this spike protein as foreign and produce natural defences’ antibodies and T cells against this protein. Later on, the vaccinated person comes into contact with SARS-CoV-2, the immune system will recognise the virus and be prepared to attack it: antibodies and T cells can work together to kill the virus, prevent its entry into the body’s cells and destroy infected cells helping to protect against COVID-19. Now ChAdOx1 nCoV-19 COVID vaccine is now used as vaccine for several health workers in India.
In conclusion, further scientific efforts are needed to evaluate the full potential of nucleoside analogues as treatment or prophylaxis of viral respiratory infections and to develop effective antivirals that are orally bioavailable and vaccines which are developed for effective against COVID-19, till now should be under observation and further judged to justify their malfunctions or bad functions on other organs in human body.
References
- Mukerjee, N (2020) A Brief Review on the Overview on Immunology of COVID-19: Current State of the Research. 9: SR201102135538,
- Johns Hopkins University. 2020. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins University, Baltimore, MD.
- National Institute of Allergy and Infectious Diseases. 2020. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. National Institute of Allergy and Infectious Diseases, Rockville, MD.W.H.O data
- Adams, E.R., Anand, R., Andersson, M.I., Auckland, K., Baillie, J.K., Barnes, E., Bell, J., Berry, T., Bibi, S., Carroll, M., et al. (2020). Evaluation of antibody testing for SARS-Cov-2 using ELISA and lateral flow immunoassays.
- Amanat, F., Stadlbauer, D., Strohmeier, S., Nguyen, T.H.O., Chromikova, V., McMahon, M., Jiang, K., Asthagiri Arunkumar, G., Jurczyszak, D., Polanco, J., et al. (2020). A serological assay to detect SARS-CoV-2 seroconversion in humans.
- Pachetti M, Marini B, Benedetti F, Giudici F, Mauro E, Storici P, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant.
- Blanco-Melo, D., Nilsson-Payant, B.E., Liu, W.-C., Uhl, S., Hoagland, D., Møller, R., Jordan, T.X., Oishi, K., Panis, M., Sachs, D., et al. (2020) Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19.
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.;Zeglen, S.; Baster, Z.; et al. (2018) Entry of human coronavirus NL63 into the cell.J. Virol,92: e01933-17.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences