Diastereoselective Catalytic Hydrogenation
Rehana Kousar*1, Naghmana Rashid2
Rehana Kousar1*, Naghmana Rashid2
1Department of Chemistry, Girls PGC Pallandri Azad Kashmir, Pakistan
2Department of Chemistry, AIOU Islamabad, Pakistan
- *Corresponding Author:
- Rehana Kousar
Department of Chemistry, Girls PGC Pallandri Azad Kashmir, Pakistan
Tel: +923009813397
E-mail: rk2389500@gmail.com
Received Date: January 29, 2021; Accepted Date: September 07, 2021; Published Date: September 17, 2021
Citation: Rehana K (2021) Diastereoselective Catalytic Hydrogenation. J Org Inorg Chem Vol.7 No.5.
Abstract
The diastereoselective catalytic hydrogenation (DCH) by heterogeneous metallic catalysts uses a covalently bound chiral auxiliary to induce the chirality. It remains an active synthetic methodology in the asymmetric synthesis of chiral products and may proceed with high diastereoselectivity. This review contains an account of previous as well as recent developments in catalytic, asymmetric processes reported for the reduction of C=C, C=O, and C=N bonds. The use of a chiral auxiliary group has also been successfully applied to the hydrogenation of aromatic and heteroaromatic rings. The choice of the chiral auxiliary was found to play a key role in the asymmetric hydrogenation. The results could be explained in terms of steric effect, with a preferred conformation of the adduct substrate and the addition occurring from the less bulky side. The catalytic metal, the support and the presence of additives were also found to have a significant influence.
Keywords
Diastereoselectivity; Hydrogenation; Heterogeneous catalysts; Chiral substrate; Chiral auxiliary
Introduction
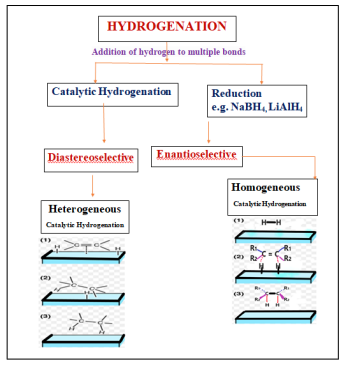
Addition of hydrogen to multiple bonds either with the application of reducing agent (reduction) or by using a catalyst (catalytic) is known as hydrogenation. Stoichiometric methods like alkali metal hydrides (LiAlH4, NaBH4, and NaCNBH3) are successfully employed for the reduction of a wide range of aldehydes and ketones [1–6]. However, their application are limited due to the stoichiometric nature of such processes, tedious work-up procedures, and the hazards associated with handling of highly reactive hydride reagents. Catalytic methods are more attractive and reactive than the stoichiometric methods, because catalyst containing reaction used for catalytic amount of species. But in stoichiometric methods, all substrates are taken at reagent level.
In particular, the use of molecular H2 as the reducing agent allows reaching 100% atom efficiency. Early hydrogenation process employed heterogeneously catalysed processes like Raney nickel, Ni and Cu chromite, which are operated at drastic conditions with temperatures in the range 200–300°C and H2 pressures of 140–300bar [7–11]. Therefore, side reactions and degradation of the reaction substrates and products may occur. With these objectives, the search for new catalyst formulations has dominated the heterogeneous catalysis research field, with the majority of works featuring novel catalyst designs. Interestingly, heterogeneous catalysts developed during the last decade share many common properties with their homogeneous counterparts. Hydrogen gas is the ideal reducing agent in terms of cost and atom efficiency, and has very broad applicability for the reduction of carbonyls. Credit goes to Wilkinson for the development of “Transfer Hydrogenation Methods” which caused an industrial revolution [12–17]. Dow pharma reported ketone hydrogenation in IPA using di (phosphine) RuCl2 (diamine) precatalysts and base as a practical alternative to NaBH4 for bulk scale. In the early 1990s, Noyori’s catalysts have rewritten the hydrogenation methods with its asymmetric hydrogenation [18–26].
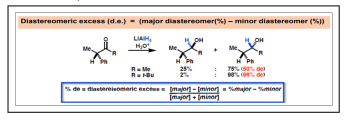
A reaction in which one of a set of stereoisomers is formed predominantly is known as stereoselective Reaction. This may be further specialized into diastereoselective or enantioselective. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The enantiomeric excess (ee) is defined as the excess of one enantiomer over the other generated in an enantioselective reaction and is usually expressed as a percentage of the whole. It usually gives a measure of the efficiency of the enantioselective reaction. The optical purity or the enantiomeric excess (ee%) of a sample can be determined as follows:
Where [R] = concentration of the R-isomer [S] = concentration of the S isomer
While a diastereoselective reaction is one in which one diastereomer is formed in preference to another, establishing a preferred relative stereochemistry. In this case, either two or more chiral centers are formed at once such that one relative stereochemistry is favored.
Mechanism of Diastereoselective Catalytic Hydrogenation
The actual pathway through which the DCH reaction proceeds may either be homogeneous or Heterogeneous.
Heterogeneous catalysis
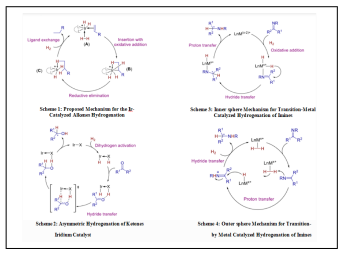
On solids, the accepted mechanism is the Horiuti-Polanyi mechanism
• Binding of the unsaturated bond, and hydrogen dissociation into atomic hydrogen onto the catalyst
• Addition of one atom of hydrogen; this step is reversible
• Addition of the second atom; effectively irreversible under hydrogenating conditions.
Homogeneous catalysis
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal (H2) complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps
• Binding of the hydrogen to give a dihydrid complex via (preceding the oxidative addition of H2 is the formation of a dihydrogen complex):
• Lnm + H2 → lnmh2
• Binding of alkene:
• Lnm(η2h2) + CH2=CHR → Ln-1MH2(CH2=CHR) + L
• Transfer of one hydrogen atom from the metal to carbon (migratory insertion)
• Ln-1MH2(CH =CHR) → Ln-1M(H)(CH2-CH2R)
• Transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
• Ln-1M(H)(CH2-CH2R) → Ln-1M + CH3-CH2R
Mechanism of Diastereoselective Catalytic Hydrogenation
Heterogeneous catalytic asymmetric hydrogenation is a powerful method for the synthesis of optically active molecules of high interest in pharmaceuticals, agrochemicals and in fragrant and flavored substance either by using enantioselective or diastereoselective route. However enantioselective route is of limited use because a very few catalytic systems have been successful, although exclusive research has been conducted in this direction.
The diastereoselective catalytic hydrogenation can be carried out by using
• Chiral auxiliary
• Chiral substrate
Chiral auxiliary is difficult to remove so chiral substrate is better to use. Various functional groups have been hydrogenated over metallic heterogeneous catalysts for the synthesis of many active compounds and this methodology has been the subject of review articles [27,28]. In present work our intention is to report the latest developments on diastereoselective hydrogenation with heterogeneous catalysts. For this purpose, we shall give an overview of few hydrogenation reactions of molecules containing C=C, C=O; and C=N bonds as well as aromatic substrates.
Diastereoselective Catalytic Hydrogenation (Dch) of C=C Bonds
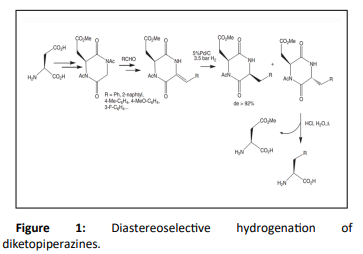
By using DCH method, Izumiya et al. [29–31] synthesized chiral dehydrodiketopiperazines from the condensation of cyclodipeptides (containing the (S)-alanine moiety) with aldehydes which on hydrolysis yield amino acid with high optical purities. Aminobutyric acid, valine, leucine, phenylalanine and tryptophane could be obtained with ee in the range of 71–99%. Cyclodipeptide was prepared by Leeming et al. [32] by using (S) - aspartic acid and was acetylated and condensed with different aromatic aldehydes to give a Z-alkene. The hydrogenation over 5% Pd/C gave the diketopiperazines in high yield with diastereoisomeric excesses >92% in favor of the cis compound. Hydrolysis led to the newly formed amino acid, which could be separated from the aspartyl unit by crystallization and pH control (Figure 1).
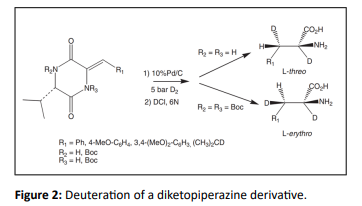
Optically active deuterated amino acids were synthesized [33] by using this DCH method as
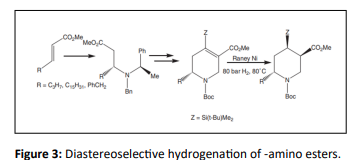
Synthesis of 2, 4, 5- trisubstituted piperidines by DCH of C=C from (R)--amino esters and methyl acrylate, was carried out via biological active valuable intermediates for synthetic compounds [34,35]. A single isomer with (2R, 4R, 5S) configuration was synthesized from DCH over Raney nickel (Figure 3).
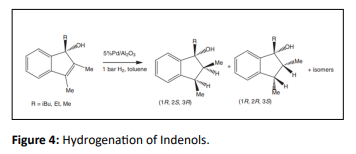
Racemic mixtures of 1-alkyl-2, 3-dimethyl indenol derivatives was obtained by the DCH of some indene derivatives on alumina-supported Pd catalysts [36] (Figure 4).
Diastereoselective Hydrogenation of C=O Bonds
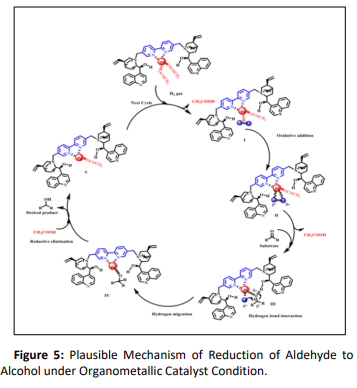
The stereoselective hydrogenation of carbonyl compounds is a very interesting reaction for the synthesis of flavour and fragrance compounds and was widely carried out by Firmenich (Figure.5) with the application of Ru complex with cinchona based chelating legands as catalyst [37]. Polysantol, dartanol and nirvanol were efficiently synthesized by DCH.
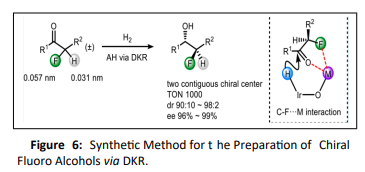
Xuefeng Tan et al with the help of Dynamic Kinetic Resolution (DKR) Strategy have developed a synthetic method for the preparation of chiral fluoro alcohols. Both high enantioselectivities and diastereoselectivities were achieved in the Ir-catalyzed hydrogenation of α-fluoro ketones via intramolecular C−F···Na interaction [Figure. 6] in the hydride transfer step which is responsible for the diastereomeric control. [39].
Rhodium complex Cy(CAAC)Rh(cod)Cl catalyzed DCH of benzene ring of indolin-2-ones (2-oxindoles) and 3,4-dihydroquinol-2-ones was carried out to a saturated cyclohexane ring with the diastereo selectivity of twenty-one hexahydroindolin-2(3H)-ones (70−99% yield, dr=83/17 to >99/1) and twelve octahydro2(1H)-quinolinones (87−96% yield, dr = 64/36 to >99/1) with the major diastereoisomer exhibiting the hydrogen atoms in an all-cis arrangement. This represent the high tolerance toward functional groups and the compatibility with existing stereogenic centers of the hydrogenation protocol [40] (Table 1).
| Diastereoselective Rhodium-Catalyzed Hydrogenation of 2âÃâ¬ÃâOxindoles and 3,4-Dihydroquinolones | |
|---|---|
| General reaction | 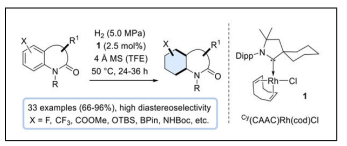 |
| Scheme-I. Diastereoselectivity of theRhCatalyzed Hydrogenation of Oxindoles | 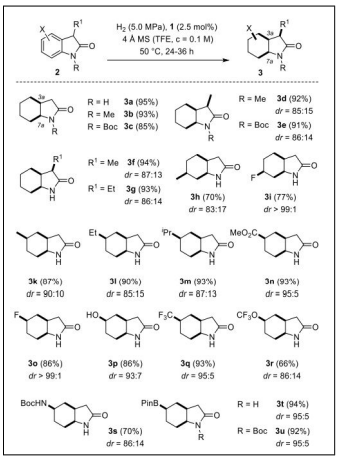 |
| SCHEME-2. Induced Diastereoselectivity (5b−5k)of the RhCatalyzed Hydrogenation of Dihydroquinolones | 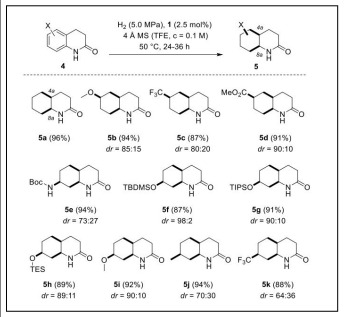 |
Table 1: Diastereoselective Rhodium-Catalysed Hydrogenation of 2 Oxindoles and 3,4-Dihydroquinolones.
Diastereoselective Hydrogenation of Aromatic Compounds
Besson et al. studied the diastereoselective hydrogenation of o-toluic acid with several chiral auxiliaries. The cis stereoisomers were formed predominantly, and the best facial differentiation was achieved using proline ester [41] and pyroglutamic acid methyl ester [42], (Figure 7).
Polycyclic aromatic hydrocarbons (PAHs) are thermodynamic stable due to aromaticity and therefore are difficult substrates for hydrogenation. Bo Han et al reported the first chromium- and cobalt-catalyzed, regiocontrolled hydrogenation of APHs at ambient temperature [43] (Table 2).
| Table.2 Hydrogenation of PAHs | |
|---|---|
| Scheme.1 Transition-Metal-Catalyzed Regioselective Hydrogenation of PAHs [43] | 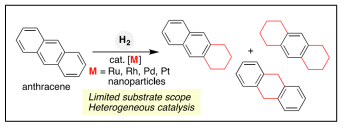 |
| Scheme 2. Chromium-Catalyzed Hydrogenation ofPAHs by Regioselective Reduction of One Terminal Carbocycle [43]. | 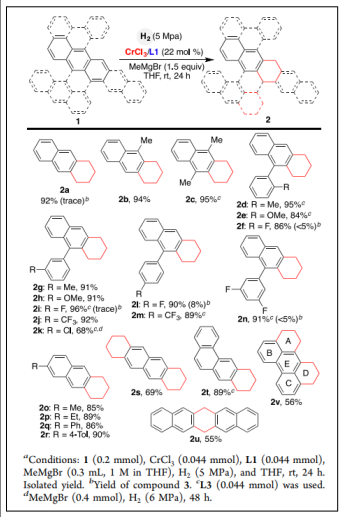 |
| Scheme 3. Cobalt-Catalyzed Hydrogenation of PAHs byRegiocontrolled Reduction of Two Terminal Carbocycle [43]. | 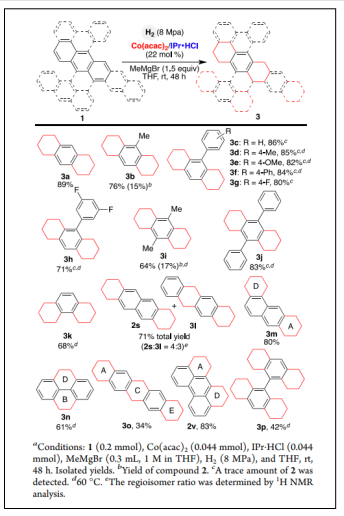 |
Table 2: Hydrogenation of PAHs
| Diels-Alder reactions were largely described [44,45]. |  |
| Hydrogenation of (S)-N-(tert-butyl)-1,2,3,4-tetrahydro-3-isoquinoline carboxamide[46]. | 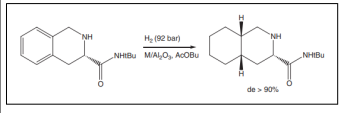 |
| Hydrogenation of monosubstituted indanes [47] . | 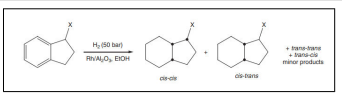 |
| Synthesis of3-PPP ((3-hydroxyphenyl)-N-(1-propyl)-piperidine) [48]. | 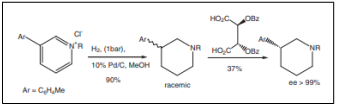 |
| Selective cis-hydrogenation of a disubstituted pyridine [49]. | 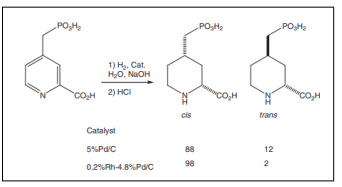 |
| Electroreduction of pyridine dicarboxylic ester [50]. | 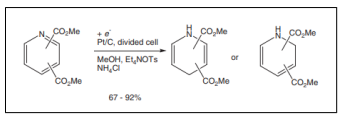 |
| Partial reduction of pyridine derivatives with borohydride[51–53]. | 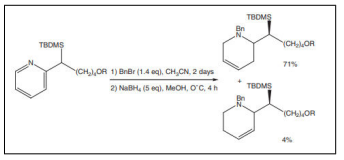 |
| Chiral auxiliaries used in diastereoselective hydrogenation of nicotinoyl derivativesl. [54] | 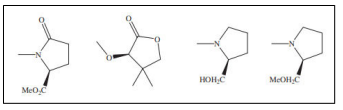 |
| Hydrogenation of N-picolinoyl-(S)-proline methyl ester [55,56]. | 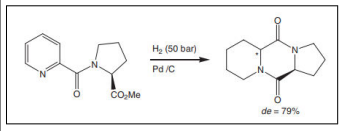 |
| Hydrogenation of 2-methyl-N-icotinoyl-(S)-proline methyl ester [54]. | 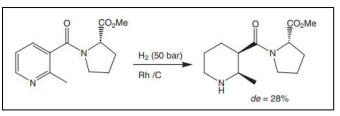 |
| Hydrogenation of pyridyl-2-phenylacetamide [57]. | 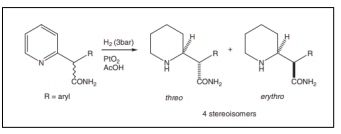 |
| Hydrogenation of a furan derivative [58]. | 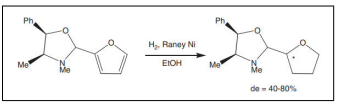 |
| Precursor of non actic acid. | 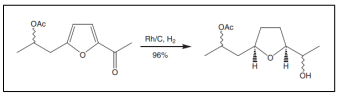 |
| Diastereoselective hydrogenation of substituted pyrrole derivatives. | 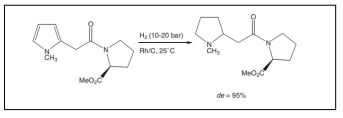 |
| Ru-catalyzed ring-closing metathesis. | 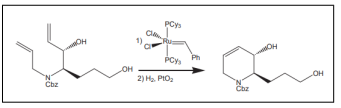 |
| Synthesis of cis-2-hydroxycyclohexylamine by hydrogenation of an imine. |  |
| Diastereoselective Hydrogenation of Chiral Imines. | 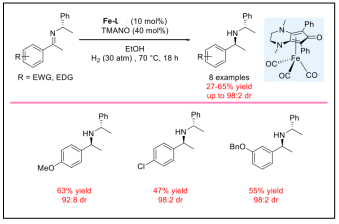 |
| Synthesis of Rasagiline and Tamsulosin Precursors | 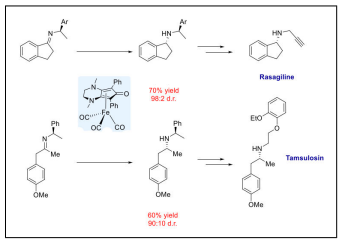 |
| Iron-Catalyzed Imine Hydrogenation | 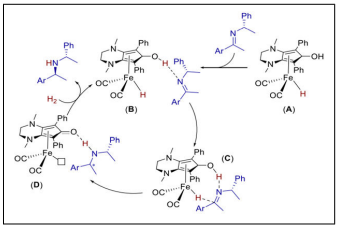 |
Table 3: Diastereoselective catalytic hydrogenation of the pyridine ring and other N-containing rings.
Industrial Applications
Catalytic hydrogenation has diverse industrial uses. Most frequently, industrial hydrogenation relies on heterogeneous catalysts.
Food industry
The largest scale application of hydrogenation is for the processing of vegetable oils. Typical vegetable oils are derived from polyunsaturated fatty acids (containing more than one carbon-carbon double bond). Hydrogenation reduces or eliminates these double bonds. The goal is to turn liquid oils into solid or semi-solid fats that can replace butter and shortening in spreads, candies, baked good and other products. Partial hydrogenation of typical plant oil to a typical component of margarine. Most of the C=C double bonds are removed in this process, which elevates the melting point of the product.
Petrochemical industry
In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. Relevant to liquid fuels that are stored sometimes for long periods in air, saturated hydrocarbons exhibit superior storage properties. On the other hand, alkenes tend to form hydroperoxides, which can form gums that interfere with fuel handling equipment. For example, mineral turpentine is usually hydrogenated. Hydrocracking of heavy residues into diesel is another application. In isomerization and catalytic reforming processes, some hydrogen pressure is maintained to hydrogenolyzecoke formed on the catalyst and prevent its accumulation.
Organic chemistry
Hydrogenation is a useful means for converting unsaturated compounds into saturated derivatives. Substrates include not only alkenes and alkynes, but also aldehydes, imines, and nitriles, which are converted into the corresponding saturated compounds, i.e. alcohols and amines. Thus, alkyl aldehydes, which can be synthesized with the oxo process from carbon monoxide and an alkene, can be converted to alcohols. E.g. 1-propanol is produced from propionaldehyde, produced from ethene and carbon monoxide. Xylitol, a polyol, is produced by hydrogenation of the sugar xylose, an aldehyde.
Primary amines can be synthesized by hydrogenation of nitriles, while nitriles are readily synthesized from cyanide and a suitable electrophile. For example, isophorone diamine, a precursor to the polyurethane monomer isophorone diisocyanate, is produced from isophorone nitrile by a tandem nitrile hydrogenation/reductive amination by ammonia, wherein hydrogenation converts both the nitrile into an amine and the imine formed from the aldehyde and ammonia into another amine.
The application of diastereoselective hydrogenation catalyzed by heterogeneous catalysts for the asymmetric synthesis of organic compounds is illustrated in the reduction of several functional groups. In that approach, the chiral information is provided by the prior attachment of a chiral auxiliary to the substrate to be hydrogenated. The optically active hydrogenated product is then disconnected from the chiral auxiliary. Proper choice of the inductor, of the catalyst and of reaction conditions may result in high diastereo selectivities.
Conclusion
The strategy of liquid-phase diastereoselective hydrogenation over a metallic catalyst is a useful method for the synthesis of many optically active compounds. Examination of the literature reveals that the diastereoselectivity is dependent on the chiral auxiliary, the catalyst (metal, support) and the solvent used. Diasterioselective heterogeneous catalytic hydrogenation involves the addition of hydrogen atoms from the catalyst surface to the adsorbed substrate molecule and the electron-rich part of the molecule approaches the metal from the least hindered side. The selectivity in the diastereoselective hydrogenation is therefore controlled by the conformation of the substrate-chiral auxiliary moiety and its adsorption on the catalyst. A high selectivity can be achieved if a strong effect of steric hindrance is exerted by the chiral auxiliary, which will allow the adsorption from one side of the reactive conformation opposite to the bulky group and at the same time prevent the adsorption from the other side. It is also important that the rotations around the bonds of connection of the chiral auxiliary are prevented. Rigid structures, eventually fixed by intramolecular hydrogen bonding to form polycyclic molecules, are favorable factors influencing the diastereoselectivity. Electronic interactions between the functional groups on the molecule and the metal surface may participate to the specific adsorption.
The metal also displays an appreciable effect on the selectivity, though it is often not predictable which metal will provide the best diastereoselectivity. Palladium is generally preferred for C=C hydrogenation, whereas rhodium and ruthenium are used for aromatic or heteroaromatic compounds. There are numerous examples, where one metal was found to afford high de, while others showed little selectivity. The extent of diastereoselectivity was also found to be dependent on the modification of the catalyst by additives (amines, tartaric acid, cinchonidine) or by the secondary or tertiary amines formed during diastereoselective hydrogenation of N-heterocycles. A priori choice of a modifier, which is not necessarily chiral, is not yet possible. The solvent displays only a small effect on diastereoselectivity in most cases. However, the solvent strongly influences the reaction rate.
References
- Gaylord NG (1956) Reduction with Complex Metal Hydrides Interscience Publishers, New York.
- Ziegler K (1952) Alumino-organic syntheses in the field of olefinic hydrocarbons. Angew. Chem 64:323-329.
- Finholt AE, Jacobson EC, Ogard AE, Thompson P (1955) Organic reductions by sodium aluminum hydride. J Am Chem Soc 77:4163.
- Metal Hydrides Inc., (1955) Technical Bulletin 502-F, Sodium Borohydride.
- Brown HC, Rao BS (1956) A New Powerful Reducing Agent—Sodium Borohydride in the Presence of Aluminum Chloride and Other Polyvalent Metal Halides1, 2. J Am Chem Soc 78:2582-2588.
- Kollonitsch J, Fuchs O, Gábor V (1954) New and known complex borohydrides and some of their applications in organic syntheses. Nature 173:125-126.
- Carleton (1930) Hydrogenation of Organic Substances, 3rd edition. Van Nostrand Company, New York, 564.
- Connor R, Adkins H (1932) Hydrogenolysis of Oxygenated Organic Compounds. J Am Chem Soc 54:4678–4690
- Carruthers W (1986) Some Modern Methods of Organic Synthesis. (3rd edition) Cambridge University Press, United Kingdom.
- Ipatieff VN (1937) Hydro-polymerization. J Am Chem Soc 59:720–722
- Richard RS, Osborn AJ (1976) Catalytic hydrogenation using cationic rhodium complexes. I. Evolution of the catalytic system and the hydrogenation of olefins. J Am Chem Soc 98:2134-2143
- Osborn JA, Wilkinson G (1967) Tris(triphenylphosphine)halorhodium(I). Inorg Synth 10:67-71
- Osborn JA, Jardine FH, Young JF, Wilkinson G (1966) The preparation and properties of tris(triphenylphosphine)halogenorhodium(I) and some reactions thereof including catalytic homogeneous hydrogenation of olefins and acetylenes and their derivatives. J Chem Soc 1711-1732
- Dong H, Du SR, Zheng XY, Lyu GM, Sun LD, Li LD, et al. (2015) Lanthanide nanoparticles: from design toward bioimaging and therapy. Chem rev 115:10725-10815
- Foubelo F, Yus M (2015) Catalytic Asymmetric Transfer Hydrogenation of Imines: Recent Advances. Chem Rec 15:907-924
- Yoshimura M, Tanaka S, Kitamura M (2014) Recent topics in catalytic asymmetric hydrogenation of ketones. Tetrahedron Lett 55:3635-3640
- Matsumura K, Zhang X, Hori K, Murayama T, Ohmiya T, et al. (2011) Practical, catalytic enantio selective hydrogenation to synthesize N-unprotected β-amino esters. Org Process Res Dev 15:1130-1137
- Bradley PA, Carroll RJ, Lecouturier YC, Moore R, Noeureuil P, et al. (2010) The synthesis of two potent β-3 adrenergic receptor agonists. Org Process Res Dev 14:1326-1336
- Hansen KB, Chilenski JR, Desmond R, Devine PN, Grabowski EJ, et al. (2003) Scalable, efficient process for the synthesis of (R)-3, 5-bistrifluoromethylphenyl ethanol via catalytic asymmetric transfer hydrogenation and isolation as a DABCO inclusion complex. Tetrahedron Asymmetry 14:3581-3587
- Miyagi M, Takehara J, Collet S, Okano K (2000) Practical synthesis of (S)-1-(3-trifluoromethyl phen yl)ethanol via Ruthenium (II)-catalyzed asymmetric transfer hydrogenation. Org Proc Res Dev 4:346-348
- Tanaka K, Katsurada M, Ohno F, Shiga Y, Oda M, et al. (2000) Practical Asymmetric Synthesis of (S)-MA20565, a Wide-Spectrum Agricultural Fungicide. Org Chem 65;432-437
- Sidler DR, Sager JW, Bergan JJ, Wells KM, Bhupathy M, et al. (1997) The enantioselective synthesis of LTD4 antagonist L-708,738. Tetrahedron Asymmetry 8:161-168
- King AO, Corley EG, Anderson RK, Larsen RD, Verhoeven TR, et al. (1993) An efficient synthesis of LTD4 antagonist L-699,392. J Org Chem 58:3731-3735
- Besson M, Pinel C (1998) Diastereoselective catalytic hydrogenation on heterogeneous metal catalysts. Top Catal 5:25-38
- Tungler A, Fodor K (1997) Synthesis of chiral amino acids and amines over solid catalysts. Catal today 37:191-208
- Laschat S, Dickner T (2000) Stereoselective synthesis of piperidines. Synthesis. 2000:1781-813
- Couty F (1999) Asymmetric syntheses of pipecolic acid and derivatives. Amino Acids 16:297-320
- Izumiya N, Lee S, Kanmera T, Aoyagi H (1997) Asymmetric hydrogenation of. alpha.,. beta.-dehydroamino acid residue in cyclic dipeptides. J Am Chem Soc 99:8346-8348
- Leeming P, Fronczek FR, Ager DJ, Laneman SA (2000) Asymmetric hydrogenations of diketopiperazines. Topic Catal 13:175-177
- Oba M, Terauchi T, Owari Y, Imai Y, Motoyama I, et al. (1998) Stereo-divergent synthesis of L-threo-and L-erythro-[2, 3-2H2] amino acids using optically active dioxopiperazine as a chiral template. J Chem Soc, Perkin Trans 1 1:1275-1282
- Ma D, Sun H (1999) Diastereoselective synthesis of 2, 4, 5-trisubstituted piperidines from enantiopure β-amino esters. Tetrahedron lett 40:3609-3612
- Ma D, Sun H. (2000) General route to 2, 4, 5-trisubstituted piperidines from enantiopure β-amino esters. Total synthesis of Pseudodistomin B triacetate and Pseudodistomin F. J Org Chem 65:6009-6016
- Chidambaram RR, Sadhasivam V, Mariyappan M, Siva A (2019) Catalytic hydrogenation of aldehydes and ketones using cinchona–bipyridyl-based palladium catalyst. J Iran Chem Soc 16:373-384
- Tan X, Zeng W, Wen J, Zhang X (2020) Iridium-Catalyzed Asymmetric Hydrogenation of α-Fluoro Ketones via a Dynamic Kinetic Resolution Strategy. Org Lett 22:7230-7233
- Schiwek CH, Jandl C, Bach T (2020) Diastereoselective Rhodium-Catalyzed Hydrogenation of 2-Oxindoles and 3, 4-Dihydroquinolones. Organic Lett 22(24):9468-9472
- Besson M, Blanc B, Champelet M, Gallezot P, Nasar K, et al. (1997) Diastereoselective hydrogenation of substituted aromatics on supported metal catalysts. Journal of Catal 170:254-264
- Malz Jr RE, editor (1996) Catalysis of Organic Reactions. CRC Press, New York
- Besson M, Neto S, Pinel C (1998) Diastereoselective hydrogenation of o-toluic acid derivatives over supported rhodium and ruthenium heterogeneous catalysts. Chem Comm 14:1431-1432
- Han B, Ma P, Cong X, Chen H, Zeng X (2019) Chromium-and cobalt-catalyzed, regiocontrolled hydrogenation of polycyclic aromatic hydrocarbons: a combined experimental and theoretical study. J Am Chem Soc 141:9018-9026
- Bailey PD, Smith PD, Morgan KM, Rosair GM. (2002) The use of the aza-Diels–Alder reaction in the synthesis of pinidine and other piperidine alkaloids. Tetrahedron let 43:1071-1074
- Kwak BS, Kim TJ, Lee SI (2002) Synthesis of (3S, 4aS, 8aS)-N-(t-butyl) decahydro-3-isoquinolinecarboxamide by diastereoselective hydrogenation over supported metal catalysts. Catalysis lett 83:93-96
- Ranade VS, Consiglio G, Prins R (2000) Functional-Group-Directed Diastereoselective Hydrogenation of Aromatic Compounds. 21. The J Org Chem 65:1132-1138
- Steiner H, Giannousis P, Pische-Jacques A, Blaser HU (2000) Diastereoselective hydrogenation of 4-(phosphonomethyl)-2-pyridinecarboxylic acid. Top Catal 13:191-194
- Kita Y, Maekawa H, Yamasaki Y, Nishiguchi I (1999) Selective and facile electroreductive synthesis of dihydro-and tetrahydropyridine dicarboxylic acid derivatives. Tetrahedron lett 40:8587-8590
- Reilly M, Anthony DR, Gallagher C (2003) Concise, enantiospecific synthesis of (3S, 4R)-3-amino-4-ethylpiperidine as partner to a non-fluoroquinolone nucleus. Tetrahedron let 44:2927-2930
- Vidal T, Magnier E, Langlois Y (1998) Formal synthesis of Manzamine C via a sila-Cope elimination. Tetrahedron 54:5959-5966
- Bates RW, Boonsombat J (2001) The pyridinium reduction route to alkaloids: a synthesis of (±)-tashiromine. J Chem SocPerkin Trans 1 7:654-656
- Douja N, Besson M, Gallezot P, Pinel C (2002) Diastereoselective hydrogenation of 2-methylnicotinic acid derivatives with supported metallic catalysts. J Mol Catal A: Chem 186:145-151
- HegedÃâ¦Ã±s L, Háda V, Tungler A, Máthé T, Szepesy L (2000) Diastereoselective heterogeneous catalytic hydrogenation of N-heterocycles. Part I. Hydrogenation of pyridines. Appl Catal A: Gen 201:107-114
- Hegedus L, Hada V, Tungler A, Máthé T, Szepesy L (2000) Diastereoselective heterogeneous catalytic hydrogenation of N-heterocycles. Part I. Hydrogenation of pyridines. Appl Catal A: Gen 201:107-114
- Ford ME (2000) Catalysis of organic reactions. (1st edition), CRC press, united states.
- Polyak F, Dorofeeva T, Zelchan G (1995) Catalytic Hydrogenation of Chiral 2-(2-Furyl)-3, 4-dimethyl-5-phenyloxazolidine. Synth commun 25:2895-2900
- Hada V, Tungler A, Szepesy L (2001) Diastereoselective heterogeneous catalytic hydrogenation of N-heterocycles: Part II. Hydrogenation of pyrroles. Appl Catal A: Gen 210:165-171
- Taniguchi T, Ogasawara K (2000) A diastereocontrolled synthesis of (+)-febrifugine: a potent antimalarial piperidine alkaloid. Organic Lett 2:3193-3195
- Kumareswaran R, Balasubramanian T, Hassner A (2000) Synthesis of enantiopure pyrrolidine and piperidine derivatives via ring closing metathesis. Tetrahedron Lett 1:8157-8162
- Lauktien G, Volk FJ, Frahm AW (1997) Diastereo-and enantioselective synthesis of cis-2-hydroxycyclohexanamine and corresponding ethers by asymmetric reductive amination. Tetrahedron: Asymmetry 8:3457-366
- Voigtmann U, Blechert S (2000) Enantioselective synthesis of α, α′-disubstituted piperidines via ruthenium-catalyzed ring rearrangement. Synthesis 2000:893-898
- Abdine RA, Hedouin G, Colobert F, Wencel-Delord J (2020) Metal-Catalyzed Asymmetric Hydrogenation of CâÃâ¢Ã N Bonds. ACS Catal 11:215-247
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences