Synthesis and Antimicrobial Activity of Benzo[H][1,6]Naphthyridine Derivatives
Raghunath BT, Balasaheb PP, Satish MC, Pratiksha G and Vasant MP
DOI10.21767/2472-1123.100009
Raghunath BT*, Balasaheb PP, Satish MC, Pratiksha G and Vasant MP
Organic Chemistry Research Centre, Department of Chemistry, KRT Arts, BH Commerce and AM Science College, Nashik, Maharashtra, India
- *Corresponding Author:
- Raghunath B Toche
Organic Chemistry Research Centre
Department of Chemistry, KRT Arts
BH Commerce and AM Science College
Affiliated to Savitribai Phule Pune University
Shivajinagar, Gangapur Road, Nashik-422 002
Maharashtra, India
Tel: +91253257641
E-mail: raghunath_toche@rediffmail.com
Received date: February 02, 2016; Accepted date: February 29, 2016; Published date: March 07, 2016
Citation: Raghunath BT, Balasaheb PP, Satish MC, et al. Synthe sis and Antimicrobial Activity of Benzo[H][1,6]Naphthyridine Derivatives. J Org Inorg Chem. 2016, 2:1.
Abstract
One pot organic reaction of 2-aminoquinoline 1 and ethyl orthoacetate with butynenitrile 2 yielded 4-amino butenenitrile quinoline 4 and with diethyl 2-(ethoxymethylene) malonate 5 yielded 4-amino methylene diethyl malonate 6 in good yield. The quinoline 6 was oxidized to quinolone 7 in acetic acid. The cyclization compound 6 was successfully attempted using Pb2O furnished 4-hydroxy benzo[h][1,6] naphthyride and on refluxing compound 6 in POCl3 yielded 4-chloro benzo[h][1,6] naphthyride derivatives 9. All new compounds showed good antimicrobial activity against standard ampicillin and streptomycin.
Keywords
Benzo[h][1,6]Naphthyridines; Methylene diethyl malonate; Antimicrobial activity
Introduction
The emerging resistance of antibacterial agents is worldwide problem proved the need of new molecules [1,2]. However, in last two decades only one new class of antibiotics has been commercialized, and there is a concerning dearth of antibacterial agents with mechanism of action in development. Bacterial fatty acid biosynthesis is an essential process that supplied precursors for the assembly of important cellular components such as phospholipids, lipoproteins, lipopolysaccharides, mycolic acids, and the cell envelope. In mammals, all enzymatic activities associated with acyl chain elongation are encoded by a single polypeptide. While in bacteria, the pathway is comprised of several discrete enzymes. This organizational difference makes the bacterial fatty acid biosynthetic enzymes potentially selective antibacterial targets [3,4]. Bacterial resistance to currently used antibiotics is becoming a concern to public health (Monroe and Polk). The development of bacterial super resistant strain is resulting in currently used antibiotic agents failing to end many bacterial infections. For this reason the search is ongoing for new natural or synthetic antimicrobial agents [5]. Quinoline derivatives have been used for the treatment of malaria [6]. Systematic modification of quinine led to the potent antimalarial chloroquine 2 drug [7]. After worldwide development, chloroquine drug was found resistant against malaria. Therefore chemist focused to synthesize new compounds. The screening test of mefloquine 3, quinacrine 4 and other new potent quinolines showed good activity against malaria [8-11]. The quinoline ring system and aliphatic side chain is crucial for the mode of action of chloroquine [12-14]. 4-Aminoquinoline bioisoster and their platinum (II) complexes showed anti-leishmanial and antitubercular activities [15]. Quinolines are structurally diverse group of compounds present in numerous natural products and are also the object of extensive synthetic research. Quinoline derivatives have demonstrated anti-leishmanial activity, antibacterial, antifungal, anti HIV and antitumor activity [16-19]. Recently, 4-amino-7- chloro-quinoline derivatives have demonstrated mycobacterium tuberculosisactivity [20-29]. Benzo[h][1,6]naphthyridine derivatives also showed antimalarial activity 5 [30].
Chemistry
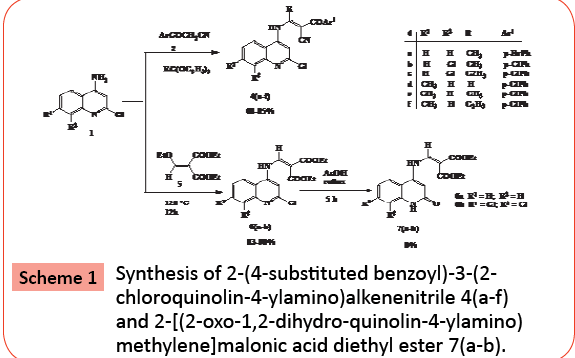
One pot condensation of p-substituted aroylacetonitriles with 2-chloroquinolin-4-amine 1 and triethylorthoester at 60-70°C in ethanol furnished amino butenenitrile quinoline derivatives 4(a-f) as yellow color solid in 70-83% yield. The structure of compounds 4 (a-f) were illustrated by spectroscopic and analytical methods. For instance IR of 4b showed the presence of NH, CN and CO stretching frequency at 3443, 2206 and 1649 cm-1 respectively. The lowering of carbonyl frequency was due to conjugation and strong intramolecular H-bonding between carbonyl oxygen and NH group. The 1H NMR spectrum of 4b in CDCl3 showed singlet at d 2.54 assignable to CH3 group; the singlet at d 7.33 assignable to C3H proton. The doublet at d 7.46 and triplet at d 7.61 (J=7.0 Hz) assignable to C7H and C6H protons respectively. The resonance singlet at d 9.95 assignable to NH proton present on secondary amino group. The remaining aromatic protons showed multiplet in between d 7.86-7.97. 13C NMR spectrum of 7b in CDCl3 showed the peaks at d 120.10 for CN group and at d 178.37 for the presence of ketone (CO). The EI-MS of 7b showed M+, M+2 and M+4 at 426, 428 and 430 m/z respectively due to the presence of two chlorine atoms [31-42]. The neat reaction of compound 1 and diethyl 2-(ethoxymethylene) malonate 5 at 120°C for 12 h furnished a yellow colored 4-amino methylene diethylmalonate 6 in 80-90% yield. The imine chloride group in compound 6 was oxidized to amide furnished derivative 7 on refluxing in acetic acid for 5 h. The structure of compound 7 was assigned using spectroscopic and analytical methods given in experimental section (Scheme 1).
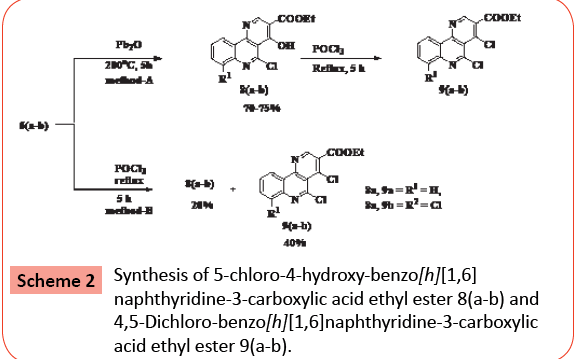
The enamine 6 was cyclized by refluxing in diphenylether for 30 min yielded brown colored to desired benzo[h][1,6]naphthyridine derivatives 8 in 70-75% yield. The compound 6a refluxed in POCl3 furnished mixture of compounds 8 and 9 as yellow coloured solids in 20 % and 40% yield respectively. The mixture of compounds 8 and 9 was separated by using column chromatography eluting with toluene. The structures of synthesized compounds were based on analytical and spectroscopic data (Scheme 2).
Study of antimicrobial activity
Cultures (Bacteria) used:
Gram positive: S. aureus, B. substilis, B. cerious, B. megaterium;
Gram negative: Escherichia coli, Pseudomonas aeruginosa, Proteus valgaris
Media used: Nutrient agar (Hi-media)
Inoculum Size: X 106 bacteria per ml
Concentration of Compound: 1000 µg/ml (prepared in DMF)
Method used: Agar diffusion assay (disc method, disc size 5 mm)
Dilution of Drug: Stock prepared 1000 µg/ml prepared in DMF [100 µg per disc]
Results of antimicrobial activity: All the synthesized compounds 4(a-f), 6(a-b), 7(a-b), 8(a-b), 9(a-b) were tested against microorganism species at 1000 ppm concentration. The observed results of antibacterial screening reported in Table 1 indicate that 4-amino substituted quinoline and benzo[h][1,6]naphthyridine compounds 4c, 4d and 7a are active against S. aureus, compounds 4e, 4f and 6a are active against E. coli bacterial species. The Compounds 4b, 4c, 4d, 4e, 6b, and 7b showed activity against B. substilis. The compounds 4d, 4f found active against B. cerious. The compounds 4c, 4d, 4f and 7b are active against B. megaterium species. However compounds 8a, 8b, 9a, 9b found totally inactive against bacterial species while the compounds 4c, 4d and 4f are most active against the bacterial species. The P. aeruginosa found stable against all compound. From the above observations it is clear that the 4-aminoquinoline derivatives 4c and 4f are showed significant antibacterial activity against B. megaterium.
| CompoundNo. | Inhibition zone diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Gram negative | Gram positive | ||||||
| E. coli | P. aeruginosa | P.vulgaris | S. aureus | B.subtilis | B. cerious | B. megaterium | |
| 4a | - | - | 11 | - | - | - | - |
| 4b | - | - | 14 | - | 8 | - | - |
| 4c | - | - | 11 | 14 | 13 | - | 18 |
| 4d | - | - | 12 | 11 | 10 | 11 | 13 |
| 4e | 10 | - | - | - | - | - | - |
| 4f | 10 | - | 12 | - | 11 | 14 | 19 |
| 6a | 8 | - | - | - | - | - | - |
| 6b | - | - | 10 | - | 8 | - | - |
| 7a | - | - | - | 8 | - | - | - |
| 7b | - | - | - | - | 8 | - | 10 |
| 8a | - | - | - | - | - | - | - |
| 8b | - | - | - | - | - | - | - |
| 9a | - | - | - | - | - | - | - |
| 9b | - | - | - | - | - | - | - |
| Ampicillin | 35 | 45 | 34 | 45 | 40 | 33 | 15 |
| Streptomycin | - | - | 34 | 13 | 15 | 40 | - |
Table 1: Antimicrobial activity of benzo[h][1,6]naphthyridine derivatives.
Experimental Section
General remarks
Melting points were determined on a Gallenkamp melting point apparatus in an open capillary tube and are uncorrected. The 1H (300 MHz) and 13C (75 MHz) NMR spectra were recorded on a Varian XL-300 spectrometer. Chemical shifts were reported in ppm relative to tetramethylsilane (TMS), and multiplicities are given as s (singlet), bs (broad singlet), d (doublet), t (triplet), q (quartet), or m (multiplet). Infrared spectra were recorded as KBr pellets on a Shimadzu FTIR-408 spectrophotometer. Mass spectra were recorded on a Shimadzu LC-MS: EI QP 2010A mass spectrometer with an ionization potential of 70eV. Elemental analyses were performed on Thermo Quest Flash 1112 Series EA analyzer. Reactions were monitored by thin layer chromatography (TLC), carried out on 0.2 mm silica gel 60 F254 (Merck) plates using UV light (254 and 366 nm) for detection and compounds were purified by column chromatography by using silica gel of 5-20 µm (Merck, 60-120 mesh). Column dimension is 39 x 2 cm and elution volume used is about 200-400 mL for each product where necessary. Common reagent-grade chemicals are either commercially available and were used without further purification or were prepared by standard literature procedures.
Synthesis of 2-(4-chlorobenzoyl)-3-(2- chloroquinolin-4-ylamino)but-2-enenitrile, 4 (a-f)
A mixture of 2-chloro-4-aminoquinoline 1 (0.005 mol), substituted benzoyl acetonitrile 2 and triethyl orthoformate or triethyl orthoacetate or triethyl orthopropionate (0.006 mol) was refluxed in dry toluene at 3 h (TLC checked, toluene). After completion of the reaction, the solvent was removed under reduced pressure; the obtained solid was stirred in methanol for 30 min. The solid separated was collected by suction filtration, dried, and recrystallized from ethanol to furnish title compound 4 in good yield.
2-(4-Bromobenzoyl)-3-(2-chloroquinolin-4-ylamino)but-2- enenitrile, 4a: Yellow prisms; Yield (1.545 g, 85%); M.P: 214°C; IR (KBr) νmax: 3353 (NH), 2924, 2230 (CN), 1640 (CO), 1539, 126 cm-1;1H NMR (CDCl3): D2.54 (s, 3H, CH3), 7.23 (s, 1H, C3H), 7.55 (t, J=7.5 Hz, 1H, C6H), 7.2 (t, J=7.5 Hz, 1H, C7H), 7.77 (d, J=7.5 Hz, 1H, C5H), 7.82-7.93(m, 5H, Ar-H), 10.15 (d, J=12.6 Hz, 1H, NH, D2O exchangeable); MS: m/z (%): 430 (M+4, 40), 428 (M+2, 50), 426 (M+, 100), 345 (30), 250 (70), 176 (80), 185 (70); Anal. Calcd. For C20H13BrClN3O (426.70); Calcd: C, 56.30; H, 3.07; N, 9.85; Found: C, 56.43; H, 2.99; N, 9.72.
2-(4-Chlorobenzoyl)-3-(2,8-dichloroquinolin-4-ylamino)but-2- enenitrile, 4b: Yellow prisms; Yield (1.46 g, 71%); M.P: 230°C; IR (KBr) νmax: ν 3443 (NH), 2924, 2206 (CN), 1649 (CO), 1575, 1280 cm-1;1H NMR (CDCl3): d 2.54 (s, 3H, CH3), 7.33 (s, 1H, C3H), 7.46 (d, J=7.0 Hz, 1H, C7H), 7.61 (t, J=7.0 Hz, 1H, C6H), 7.86-7.97(m, 5H, Ar-H), 9.95 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): d 22.32, 83.23, 110.48, 117.10, 120.07, 122.86, 123.06, 123.17, 123.26, 128.84, 130.05, 130.16, 130.87, 139.51, 148.06, 161.37, 178.37; MS: m/z (%): 422 (M+6, 20), 420 (M+, 20), 418 (M+2, 30), 416 (M+, 100), 212 (30), 204 (70), 141 (40); Anal. Calcd. For C20H12Cl3N3O (416.70); Calcd: C, 57.65; H, 2.90; N, 10.08; Found: C, 57.43; H, 2.99; N, 10.21.
2-(4-Chlorobenzoyl)-3-(2,8-dichloroquinolin-4-ylamino)-pent-2- enenitrile, 4c: Yellow prisms; Yield (1.50 g, 70%); M.P: 217°C; IR (KBr) νmax: ν 3337 (NH), 2817, 2223 (CN), 1649 (CO), 1570, 1270 cm-1;1H NMR (CDCl3): d 1.31 (t, J=7 Hz, 3H, CH3), 2.74 (q, J=7.0 Hz, 2H, CH2), 7.37 (s, 1H, C3H), 7.45 (d, J=7.5 Hz, 1H, C7H), 7.59 (t, J=7.5 Hz, C6H) 7.61-7.91(m, 5H, Ar-H), 9.87 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): d 15.28, 21.21, 79.52, 111.13, 116.23, 120.17, 122.70, 123.03, 123.30, 124.07, 127.6, 130.09, 130.71, 132.20, 140.21, 146.30, 160.20, 177.21; Anal. Calcd. For C21H14Cl3N3O (430.72); Calcd: C, 58.56; H, 3.28; N, 9.76; Found: C, 58.43; H, 3.19; N, 9.6.
2-(4-Chlorobenzoyl)-3-(2-chloro-7-methyl-quinolin-4-ylamino) acrylonitrile, 4d: Yellow prisms; Yield (1.42 g, 75%); M.P: 26°C; IR (KBr) νmax: 3475 (NH), 2908, 2215 (CN), 1645 (CO), 1570, 1170 cm-1;1H NMR (CDCl3): d 2.51 (s, 3H, CH3), 7.32 (s, 1H, C3H), 7.45 (d, J=7.0 Hz, 1H, C6H), 7.60 (d, J=7.0 Hz, C5H) 7.65-7.96(m, 5H, Ar- H), 8.21(d, J=12.5 Hz, 1H,=CH), 10.08 (d, J=12.5 Hz, 1H, NH, D2O exchangeable); MS: m/z (%): 385 (M+4, 20), 383 (M+2, 30), 381 (M+, 100), 246 (30), 192 (70), 190 (70), 139 (30); Anal. Calcd. For C20H13Cl2N3O (382.25); Calcd: C, 62.84; H, 3.43; N, 10.99; Found: C, 62.83; H, 3.59; N, 10.83.
2-(4-Chlorobenzoyl)-3-(2-chloro-7-methyl-quinolin-4-ylamino) but-2-enenitrile, 4e: Yellow prisms; Yield (1.28 g, 65%); M.P: 183°C; IR (KBr) νmax: ν 3237 (NH), 3008, 2205 (CN), 1647 (CO), 1545, 1169 cm-1;1H NMR (CDCl3): d 2.51 (s, 3H, CH3), 2.631 (s, 1H, Ar-CH3), 7.33 (s, 1H, C3H), 7.46 (d, J=7.5 Hz, 2H, ArH), 7.61 (d, J=7.5 Hz, C6H) 7.86-7.97 (m, 4H, Ar-H), 10.08 (s, 1H, NH, D2O exchangeable); Anal. Calcd. For C21H15Cl2N3O (396.28); Calcd: C, 63.65; H, 3.82; N, 10.60; Found: C, 63.69; H, 3.79; N, 10.55.
2-(4-Chlorobenzoyl)-3-(2-chloro-7-methyl-quinolin-4-ylamino) pent-2-enenitrile, 4f: Yellow prisms; Yield (1.22 g, 60%); M.P: 178°C; IR (KBr) νmax: 3337 (NH), 2917, 2215 (CN), 1645 (CO), 1570, 1170 cm-1;1H NMR (CDCl3): d 1.31 (t, J=8.0 Hz, 3H, CH3), 2.61 (s, 3h, ArCH3), 2.74 (q, J=8.0 Hz, 2H, CH2), 7.35 (s, 1H, C3H), 7.45 (d, J=8.0 Hz, 1H, ArH), 7.45 (d, J=7.5 Hz, 1H, C6H), 7.59-7.91(m, 5H, Ar-H), 10.17 (s, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): d 16.21, 23.17, 80.20, 111.31, 118.10, 120.14, 122.86, 123.09, 123.28, 123.84, 130.13, 130.94, 132.34, 139.25, 148.08, 163.30, 177.31; MS: m/z (%): 413 (M+4, 10), 411 (M+2, 30), 409 (M+, 100), 274 (30), 218 (70), 193 (80), 139 (40); Anal. Calcd. For C22H17Cl2N3O (410.31); Calcd: C, 64.40; H, 4.18; N, 10.24; Found: C, 64.71; H, 4.10; N, 10.35.
Synthesis of 2-[(2-chloroquinolinin-4-ylamino) methylene]malonic acid diethyl ester (6a-b)
2-Chloro-4-aminoquinoline 1(a-b) (0.005 mol) and diethoxymethylene malonate 5 (0.007 mol) was stirred at 120- 130°C for 12 h. Progress of the reaction was monitored by TLC. After cooling the reaction mixture to room temperature, methanol (50 mL) was added to it. The crude product separated was collected by suction filtration, dried, and recrystallized from the ethanol and DMF (8:2) to furnish yellow solid 6 in 83-90% yields.
2-[(2-Chloro-quinolinin-4-ylamino)methylene]malonic acid diethyl ester (6a): Yellow prisms; Yield (1.56 g, 90%); M.P: 205°C; IR (KBr) νmax: 3433 (NH), 2931, 1693 (CO), 1618, 1267, 806 cm- 1; 1H NMR (CDCl3): d 1.37 (t, J=7.0 Hz, 3H, CH3), 1.42 (t, J=7.0 Hz, 3H, CH3), 4.29 (q, J=7.0 Hz, 2H, CH2), 4.32 (q, J=7.0 Hz, 2H, CH2), 7.24 (s, 1H, C3H), 7.62 (t, J=6.5 Hz, 1H, C7H), 7.77 (t, J=6.5 Hz, 1H, C6H), 7.79 (d, J=6.5 Hz, 1H, C8H), 8.04 (d, J=6.5 Hz, 1H, C5H), 8.57 (d, J=12.6 Hz, 1H,=CH), 11.95 (d, J=12.6 Hz, 1H, NH, D2O exchangeable); 13C NMR (CDCl3): d 14.20, 21.71, 169.30, 109.31, 122.23, 122.91, 123.20, 132.21, 133.02, 133.70, 140.20, 145.03, 159.01, 169.21; MS: m/z (%): 350 (M+2, 30), 348 (M+, 90), 278 (20), 178 (70), 170 (70); Anal. Calcd. For C17H17ClN2O4 (348.79); Calcd: C, 58.54; H, 4.91; N, 8.03; Found: C, 58.63; H, 4.87; N, 8.15.
2-[(2,8-Dichloro-quinolinin-4-ylamino)methylene]malonicacid diethyl ester (6b): Yellow prisms; Yield (1.58 g, 83%); M.P: 183°C; IR (KBr) νmax: 3345 (NH), 2935, 1690 (CO), 1620, 1267, 870 cm- 1;1H NMR (CDCl3): d 1.35 (t, J=7.2 Hz, 3H, CH3), 1.43 (t, J=7.2 Hz, 3H, CH3), 4.30 (q, J=7.2 Hz, 2H, CH2), 4.32 (q, J=7.2 Hz, 2H, CH2), 7.25 (s, 1H, C3H), 7.60 (t, J=7.0 Hz, 1H, C6H), 7.78 (d, J=7.0 Hz, 1H, C7H), 7.95 (d, J=7.0 Hz, 1H, C5H), 8.56 (d, J=13.5 Hz, 1H,=CH), 11.95 (d, J=13.5 Hz, 1H, NH, D2O exchangeable); Anal. Calcd. For C17H16Cl2N2O4 (383.23); Calcd: C, 53.28; H, 4.21; N, 7.31; Found: C, 53.20; H, 4.27; N, 7.41.
Synthesis of 2-[(2-oxo-1,2-dihydro-quinolin-4- ylamino)methylene]malonic acid diethyl ester, 7a-b
The open chain compound 6 was refluxed in acetic acid for 5 h (TLC check, toluene). Reaction mixture was cooled to room temperature; the solid separated was collected by suction filtration, dried, and recrystallized from ethanol/DMF (8:2) to afford 7 in 80-82% yield.
2-[(2-Oxo-1,2-dihydroquinolin-4-ylamino)methylene]malonic acid diethyl ester,7a: Yellow prisms; Yield (1.32 g, 80%); M.P: 230°C; IR (KBr) νmax: 3439 (NH), 3281 (NH), 2980, 1724(CO), 1699 (CO)cm-1;1H NMR (CDCl3): d 1.27 (t, J=7.0 Hz, 3H, CH3), 1.29 (t, J=7.0 Hz, 3H, CH3), 4.14 (q, J=7.0 Hz, 2H, CH2), 4.26 (q, J=7.0 Hz, 2H, CH2), 6.52 (s, 1H, C3H), 7.31 (t, J=7.2 Hz, 1H, C6H), 7.37 (t, J=7.7 Hz, 1H, C7H), 7.56-7.60 (m, 2H, C5H& C8H), 8.49 (d, J=12.3 Hz,=CH), 11.21 (d, J=12.3 Hz, NH, 1H, NH, D2O exchangeable); Anal. Calcd. For C17H18N2O5 (330.34); Calcd: C, 61.81; H, 5.49; N, 8.48; Found: C, 61.78; H, 5.30; N, 8.51.
2-[(8-Chloro-2-oxo-1,2-dihydroquinolin-4-ylamino)methylene] malonic acid diethyl ester, 7b: Yellow prisms; Yield (1.49 g, 82%); M.P: 197°C; IR (KBr) νmax: 3433 (NH), 3270 (NH), 2982, 1725(CO), 1685 (CO) cm-1;1H NMR (CDCl3): d 1.28 (t, J=7.2 Hz, 3H, CH3), 1.30 (t, J=7.2 Hz, 3H, CH3), 4.15 (q, J=7.2 Hz, 2H, CH2), 4.26 (q, J=7.2 Hz, 2H, CH2), 6.55 (s, 1H, C3H), 7.38 (t, J=7.5 Hz, 1H, C6H), 7.56 (d, J=7.5 Hz, 1H, C7H), 7.75 (d, J=7.5 Hz, 1H, C5H), 8.47 (d, J=14 Hz,=CH), 11.18 (d, J=14 Hz, NH, 1H, NH, D2O exchangeable);MS: m/z (%): 366 (M+2, 30), 364 (M+, 90), 304 (40), 244 (60), 194 (70), 170 (80); Anal. Calcd. For C17H17ClN2O5 (364.79); Calcd: C, 55.97; H, 4.70; N, 7.68; Found: C, 55.89; H, 4.75; N, 7.69.
Synthesis of 5-chloro-4-hydroxy-benzo[h][1,6] naphthyridine-3-carboxylic acid ethyl ester, 8 (ab) and 4,5-Dichloro-benzo[h][1,6]naphthyridine- 3-carboxylic acid ethyl ester, 9 (a-b)
Method A: The compound 6 (0.005 mol) in diphenyl ether was refluxed (200°C) for 30 min (TLC checked, toluene). After completion of reaction, the reaction mixture was cool to room temperature and then it was stirred in diethyl ether (50 mL). The solid obtained was filtered and washed with excess ether, dried, and recrystallized from ethanol/DMF (80:20) to afford 8 in70-75% yield.
Method B: The compound 6 (0.005 mol) in POCl3 was heated to reflux for 5 h (TLC checked, toluene). After completion of reaction, excess POCl3 vacuum evaporated. The red colored residue obtained was poured in ice water (1L) and solution was neutralized with solid sodium carbonate (10 g). The separated solid product was collected by suction filtration. The TLC analysis in toluene showed two products. The mixture of crude product was separated by column chromatography on silica gel eluting with toluene, yields title compound 8 (20%) and 9 (40%).
5-Chloro-4-hydroxybenzo[h][1,6]naphthyridine-3-carboxylic acid ethyl ester, 8a: Yellow prisms; Yield; (Method A=1.05 g, 70%, B=0.302 g, 20%); M.P: 250°C; IR (KBr) νmax: 3423 (OH), 3050, 1720 (NH), 2982, 1720 (CO), 1624, 1566 cm-1;1H NMR (CDCl3): d 1.30 (t, J=7.2 Hz, 3H, CH3), 4.26 (q, J=7.2 Hz, 2H, CH2), 7.57 (t, J=7.5 Hz, 1H, C9H), 7.78 (t, J=7.5 Hz, 1H, C8H), 8.05 (d, J=7.5 Hz, 1H, C10H), 8.35 (d, J=7.5 Hz, 1H, C7H), 10.25 (s, 1H, OH, D2O exchangeable); Anal. Calcd. For C15H11ClN2O3 (302.72); Calcd: C, 59.52; H, 3.66; N, 9.25; Found: C, 59.60; H, 3.61; N, 9.40.
5,7-Dichloro-4-hydroxy-benzo[h][1,6]naphthyridine-3- carboxylic acid ethyl ester, 8b: Yellow prisms; Yield; (Method A=1.17 g, 75%; B=0.337 g, 20% ); M.P: 243°C; IR (KBr) νmax: 3425 (OH), 3055, 1714 (CO), 1624, 1516, 2280, 769 cm-1;1H NMR (CDCl3): d 1.31 (t, J=7.0 Hz, 3H, CH3), 4.25 (q, J=7.0 Hz, 2H, CH2), 7.77 (t, J=8.1 Hz, 1H, C9H), 8.10 (d, J=8.1 Hz, 1H, C8H), 8.42 (s, 1H, C2H), 8.54 (d, J=8.1 Hz, 1H, C10H), 10.80 (s, 1H, OH, D2O exchangeable);MS: m/z (%): 341 (M+4, 20), 339 (M+2, 30), 337 (M+, 90), 300 (70), 255 (70), 185 (80); Anal. Calcd. For C15H10Cl2N2O3 (337.16); Calcd: C, 53.44; H, 2.99; N, 8.31; Found: C, 53.50; H, 3.09; N, 8.40.
4,5-Dichlorobenzo[h][1,6]naphthyridine-3-carboxylic acid ethyl ester, 9a: Yellow prisms; Yield; (Method A=0.642 g, 40%); M.P: 188°C; IR (KBr) νmax: 2955, 1728 (CO), 156, 1174, 770 cm-1;1H NMR (CDCl3): d 1.48 (t, J=6.9 Hz, 3H, CH3), 4.51 (q, J=6.9 Hz, 2H, CH2), 7.76 (t, J=7.8 Hz, 1H, C9H), 7.88 (t, J=7.8 Hz, 1H, C8H), 8.05 (d, J=7.8 Hz, 1H, C10H), 9.05 (d, J=7.8 Hz, 1H, C7H), 9.12 (s, 1H, C2H); MS: m/z (%): 325 (M+4, 10), 323 (M+2, 30), 321 (M+, 100), 284 (30), 187 (40); Anal. Calcd. For C15H10Cl2N2O2 (321.17); Calcd: C, 56.10; H, 3.14; N, 8.72; Found: C, 56.22; H, 3.09; N, 8.69.
4,5,7-Trichlorobenzo[h][1,6]naphthyridine-3-carboxylic acid ethyl ester, 9b: Yellow prisms; Yield; (Method A=0.71 g, 40%); M.P: 149°C; IR (KBr) νmax: 2923, 160 (CO), 1537, 1179, 770 cm- 1;1H NMR (CDCl3): d 1.48 (t, J=7.0 Hz, 3H, CH3), 4.52 (q, J=7.0 Hz, 2H, CH2), 7.70 (t, J=7.2 Hz, 1H, C9H), 7.80 (d, J=7.2 Hz, 1H, C8H), 8.05 (d, J=7.2 Hz, 1H, C10H), 9.10(s, 1H, C2H); Anal. Calcd. For C15H9Cl3N2O2 (355.61); Calcd: C, 50.66; H, 2.55; N, 7.88; Found: C, 50.79; H, 2.61; N, 7.79.
Conclusion
The chemoselective synthesis of 4-aminoquinolines 6(a-b) were obtained from 2,4-dichloroquinoline derivatives at different reaction conditions. The N-alkylation on 4-aminoquinolines using benzoylacetonitrile, diethyl 2-(ethoxymethylene) malonate was carried under mild reaction conditions. The benzo[h][1,6] naphthyridine derivatives 8(a-b), 9(a-b) were obtained from open chain analog of 4-aminoquinolines 6(a-b). The open chain quinline derivatives showed antimicrobial activity while cyclic benzo[h][1,6]naphthyridine derivatives found totally against bacteria utilized.
Acknowledgements
Authors thank UGC, New Delhi and BCUD, Savitribai Phule Pune University for financial support, CIF, Department of Chemistry, Savitribai Phule Pune University for spectral analysis and Principal, KTHM College, Nashik-422 002, Maharashtra for providing facilities.
References
- Alice MRB, Alexandre RA, Luiz CSP, Júlio CB, Izabel CPP, et al. (2012) Synthesis and anti-HSV-1 evaluation of new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines and 3 H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. Organic and Medicinal Chemistry Letters 2-3: 2-8.
- Kullmann J, Dalhoft A, Zeiler HJ (1998) Quinoline as antibacterials. 1st edn. Spinger-Verlag, Berlin.
- David J (2002) Discovery of a Novel and Potent Class of FabI-Directed Antibacterial Agents. Antimicrobial Agents and Chemotherapy 46: 3118-3124.
- Fritz HK, Kurt AB, Johannes E, Rolf MZ (1998) Medical Microbiology. Stuttgart, New York, NY: Georg Thieme Verlag.
- O'Hara CM (2000) Classification, identification, and clinical significance of Proteus. Providencia and Morganella. Clin Micro biolRev13: 534-546.
- Coatney GR, Cooper WC, Eddy NB (1953) Greenberg. J Public Health Monogr, pp: 151-322.
- Rieckmann KH, Trenholme GM, Williams RL, Carson PE, Frischer H, et al. (1974) Prophylactic activity of mefloquine hydrochloride (WR 142 490) in drug-resistant malaria. Bull World Health Organ 51: 375-377.
- O’Neill PM, Mukhtar A, Stocks PA, Randle LE, Hindley S, et al. (2003) Isoquine and Related Amodiaquine Analogues: A New Generation of Improved 4-Aminoquinoline Antimalarials. J Med Chem 46: 4933-4945.
- De D, Krogstad FM, Cogswell FB, Krogstad DJ (1996) Aminoquinolines That Circumvent Resistance in Plasmodium falciparum in Vitro. Am J Trop Med Hyg 55: 579-583.
- Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A, et al. (1996) 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob. Agents Chemother 40: 1846-1854.
- Stocks PA, Raynes KJ, Bray PG, Park BK, O’Neill PM, et al. (2002) Novel Short Chain Chloroquine Analogues Retain Activity against Chloroquine Resistant K1 Plasmodium falciparum. J Med Chem 45: 4975-4983.
- Srinivasa RC, Maiti S, Dorn A, Scorneaux B, Bhattacharjee AK (2003) Carbon Isosteres of the 4-Aminopyridine Substructure of Chloroquine: Effects on pKa, Hematin Binding, Inhibition of Hemozoin Formation, and Parasite Growth. J Med Chem 46: 3166-3169.
- Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A (2000) Structure−Function Relationships in Aminoquinolines: Effect of Amino and Chloro Groups on Quinoline−Hematin Complex Formation, Inhibition of β-Hematin Formation, and Antiplasmodial Activity. J Med Chem 43: 283-291.
- Kaschula CH, Egan TJ, Hunter R, Basilico N, Parapini S, et al. (2002) Structure−Activity Relationships in 4-Aminoquinoline Antiplasmodials. The Role of the Group at the 7-Position. J Med Chem45: 3531-3539.
- Carmo AML, Silva FMC, Machado PA, Fontes APS, Pavan FR, et al. (2011) Synthesis of 4-aminoquinoline analogues and their platinum(II)complexes as new antileishmanial and antitubercular agents. Biomedicine & Pharmacotherapy 65: 204-220.
- Strekowski L, Mokrosz JL, Honkan VA, Czarny A, Cegla MT, et al. (1991) Synthesis and quantitative structure-activity relationship analysis of 2-(aryl or heteroaryl)quinolin-4-amines, a new class of anti-HIV-1 agents. J Med Chem 34: 1739-1746.
- Abel MD, Ha CM, Luu HT, Micetich RG, Nguyen DQ, et al. (1996) Cytotoxic quinolines (Part 1) Azolylalkoxyquinolines and azolylalkyl-4(1H)- quinolines. Drug Des Discov 14: 15-30.
- Rasoul-Amini S, Khalaj A, Shaffie A, Daneshtalab M, Madadkar-Sobhani A, et al. (2006) Anti-tumor Activity of New Quinoline Derivatives in Human Breast Cancer T47D Cells.Int J Canc Res 2: 102-108.
- De D, Krogstad FM, Byers LD, Krogstad DJ (1998) Structure−Activity Relationships for Antiplasmodial Activity among 7-Substituted 4-Aminoquinolines. J Med Chem 41: 4918-4926.
- Vieira LM, Almeida MV, Lourenco MC, Bezerra FA, Fontes APS (2009) Synthesis and antitubercular activity of palladium and platinum complexes with fluoroquinolones. European Journal of Medicinal Chemistry 44: 4107-4111.
- Kinnamon K, Steck EA, Rane DS (1979) Activity of Antitumor Drugs against African Trypanosomes. Antimicrob. Agents Chemother 15: 157-160.
- Visbal G, Marchan E, Maldonado A, Simoni Z, Navarro M (2008) Synthesis and characterization of platinum–sterol hydrazone complexes with biological activity against Leishmania (L.) Mexicana. Journal of Inorganic Biochemistry 102: 547-554.
- Pavan FR, Vonpoelhsitz G, Do Nascimento F, Leite SRA, Batista AA, et al. (2010) Ruthenium (II) phosphine/picolinate complexes as antimycobacterial agents. European Journal of Medicinal Chemistry 45: 598-601.
- Maia PIS, Pavan FR, Leite CQF, Lemos SS, Souza GF, et al. (2009) Vanadium complexes with thiosemicarbazones: Synthesis, characterization, crystal structures and anti-Mycobacterium tuberculosis activity. Polyhedron 28: 398-406.
- Moro C, Mauro AE, Netto VGN, Ananias SR, Quilles MB, et al. (2009) Antitumor and antimycobacterial activities of cyclopalladated complexes: X-ray structure of [Pd(C2,N-dmba)(Br)(tu)] (dmba = N,N-dimethylbenzylamine, tu = thiourea. Eur J Med Chem 44: 4611-4615.
- Do Nascimento F, Poelhsitz GV, Pavan FR, Sato DN, Leite CQF, et al. (2008) Synthesis, characterization, X-ray structure and in vitro antimycobacterial and antitumoral activities of Ru(II) phosphine/diimine complexes containing the “SpymMe2” ligand, SpymMe2 = 4,6-dimethyl-2-mercaptopyrimidine. J Inorg Biochem 102: 1783-1789.
- Tarallo MB, Costa-Filho AJ, Vieira ED, Monge A, Leite CQF, et al. (2009) Anales de ia. Asociation Quimica Argentina 97: 80-89.
- Keith CT, Borisy AA, Stockwell BR (2005) Multicomponent therapeutics for networked systems. Nature Reviews Drug Discovery 4: 71-78.
- Acharya BN, Saraswat D, Tiwari M, Shrivastava AK, Ghorpade R, et al. (2010) Synthesis and antimalarial evaluation of 1, 3, 5-trisubstituted pyrazolines. Eur J Med Chem 45: 430-438.
- Sullivan DJ (2002) Theories on malarial pigment formation and quinoline action. Int J Parasitol 32: 1645-1653.
- Roseman KA, Gould MM, Linfield WM, Edwards BE (1970) Antimalarials. 8-chloro-4- (2'-N,N-dibutylamino-1'-hydroxyethyl) benzo[h]-1,6-naphthyridine. J Med Chem 13: 230-233.
- Bax R, Mullan N, Verhoef J (2000) The millennium bugs--the need for and development of new antibacterials. Int J Antimicrob Agents 16: 51-59.
- Setti EL, Quattrocchio L, Micetich RG(1997) Current approaches to overcome bacterial resistance. Drugs of the Future 22:271-284.
- Toche RB, Pagar BP, Zoman RR, Shinde GB, Jachak MN (2010) Synthesis of novel benzo[h][1,6]naphthyridine derivatives from 4- aminoquinoline and cyclic b-ketoester. Tetrahedron 66: 5204-5211.
- Manoj M, Rajendra Prasad KJ (2010) Synthesis of Novel Alkyl and Aryl Substituted Dibenzo[b,h][1,6]naphthyridines. Synthetic Communications 42: 434-446.
- Prabha K, Rajendra Prasad KJ (2012) Synthesis of Alkyl and Aryl Substituted Benzo[h]Naphtho[1,2-b][1,6]naphthyridines. Synthetic Communications 42: 2277-2289.
- Abdel-Wahab BF, Khidre RE (2013) 2-Chloroquinoline-3-carbaldehyde II: Synthesis, Reactions, and Applications. Journal of Chemistry, p: 13.
- Kolandaivel P, Rajendra Prasad KJ (2015) Benzoquinoline amines – Key intermediates for the synthesis of angular and linear dinaphthonaphthyridines. Journal of Advanced Research 6: 631-641.
- Isravel M, Perumal V, Thavaraj V, Nagarajan S, Uma Maheswari C, et al. (2015) Synthesis of 5,6-Dihydrodibenzo[b,h][1,6]naphthyridines via Copper Bromide Catalyzed Intramolecular [4+2]Hetero-Diels–Alder Reactions.J Org Chem, p: 22.
- Shaheen F, Abha B, Anil KV (2015) Synthesis, spectral analysis (FT-IR, 1H NMR, 13C NMR and UV–visible) and quantum chemical studies on molecular geometry, NBO, NLO, chemical reactivity and thermodynamic properties of novel 2-amino-4-(4-(dimethylamino)phenyl)-5-oxo-6-phenyl-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile. Journal of Molecular Structure 1095: 112-124.
- Afaf MAH (2015) Rapid synthesis of 1,6-naphthyridines by grindstone chemistry. Environmental Chemistry Letters 13: 125-129.
- Dao-Lin W, Dan W, Wei Z, Yong-Yang W, Jian-Ying W (2015) An efficient synthesis of benzo[b]benzofurano[2,3-e]- [1,6]naphthyridine-8-ones. Chinese Chemical Letters 26: 251-254.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences